eISSN: 2373-6372


Review Article Volume 4 Issue 6
Department of Surgery, Federal University of Minas Gerais, Brazil
Correspondence: Luiz Ronaldo Alberti, Adjunct Professor, Department of Surgery, Faculty Researcher at Santa Casa Hospital in Belo Horizonte, UFMG, Gastroenterologist and Endoscopist, Rua do Ouro 1170, apto 1200, CEP 30220-000, Belo Horizonte, Minas Gerais, Brazil, Tel 55-(31)-99955-0400
Received: April 21, 2016 | Published: June 20, 2016
Citation: Alberti LR, Santos RS, Castro AFD, Fonseca APDFP, Key E, et al (2016) Upper Gastrointestinal Bleeding in Oncological Patients. Gastroenterol Hepatol Open Access 4(6): 00123. DOI: 10.15406/ghoa.2016.04.00123
Bleeding is a relatively common phenomenon in patients with solid neoplasms, occurring in up to 10% of all patients in advanced stages of cancer. Therefore, this study aims to discuss clinical support management, endoscopic strategies, radiotherapy, chemotherapy, as well as endovascular and surgical interventions.
Bleeding is a relatively common phenomenon in patients with solid neoplasms, occurring in up to 10% of all patients in advanced stages of cancer. The objective of this article is to review the etiology, clinical manifestations, and treatment of acute upper gastrointestinal bleeding in cancer patients, understanding that chronic and occult blood losses are not within the scope of this text. To achieve better outcomes in the management of this entity, a multidisciplinary approach is mandatory. Therefore, this study aims to discuss clinical support management, endoscopic strategies, radiotherapy, chemotherapy, as well as endovascular and surgical interventions.
Keywords: upper gastrointestinal bleeding, bleeding, neoplasms, endoscopy, treatment, critical care
NSAID, non-steriodal anti-inflammatory drug; HAN, hydroaerial noises; UGE, upper gastrointestinal endoscopy; CVP, central venous pressure; IAP, intra-arterial pressure; PPIs, proton-pump inhibitors; ASA, acetylsalicylic acid
Bleeding is a relatively common phenomenon in patients with solid neoplasms, occurring in up to 10% of all patients in advanced stages of cancer.1 This condition can cause serious morbidity and even death. Upper gastrointestinal bleeding is defined as a bleeding resulting from a site proximal to the Treitz ligament. In an observational prospective study of patients either being admitted to the hospital or during their hospitalization in acute palliative care units, upper sources of bleeding were present in 7% of the cases.2 In another prospective cohort, bleeding was also present in 5% of the cancer patients in the last three days of their lives.3 The objective of this article is to review the etiology, clinical manifestations, and treatment of acute upper gastrointestinal bleeding in cancer patients, understanding that chronic and occult blood losses are not within the scope of this text. To achieve better outcomes in the management of this entity, a multidisciplinary approach is mandatory. Therefore, this study aims to discuss clinical support management, endoscopic strategies, radiotherapy, chemotherapy, as well as endovascular and surgical interventions.
The main causes of upper gastrointestinal bleeding in patients with cancer is a matter of debate. A prospective study of 65 oncologic patients conduced at the Memorial Sloan-Kettering Cancer Center found that hemorrhagic gastritis was the main cause of bleeding, present in 40% of the patients, followed by benign peptic ulcer disease in 22% of the cases.4 In the same series, gastric cancer was seen in 28% of the patients, although it was considered the source of bleeding in only 15%. Many authors argue that the etiology of upper gastrointestinal bleeding in oncological patients is the same as in the general population, with peptic ulcers most commonly caused by acute blood loss, esophageal and gastric varices, and esophagitis and erosive lesions.5 In this context, acute bleeding stemming directly from the tumor itself would be a less common event (2.9% to 4% of all cases), since these patients commonly present chronic blood loss.6 Chemotherapy, nonsteroidal anti-inflammatory drugs, and anticoagulants are potential agents that can contribute to erosive lesions and bleeding in cancer patients. In patients with cirrhosis, one population based study also claims that variceal bleeding corresponds to a significant proportion of the cases (50%-60%).7 Cancer patients can also present portal hypertension and esophageal or gastric varices caused by diffuse liver infiltration, portal vein thrombosis, or hepatic vein thrombosis (Budd-Chiari syndrome). In many cases, they exhibit a state of hypercoagulability that is predisposed to thrombosis. Thus, portal hypertension with varices must be included in the potential sources of bleeding in oncological patients even if they do not present cirrhosis or diffuse liver infiltration.8
One large retrospective database study of 240,428 endoscopies performed in a wide range of clinics to search for the cause of upper gastrointestinal bleeding (variceal bleeding was excluded from the analysis) revealed that peptic ulcers were present in 32.7% of the patients, followed by erosion in 18.8% of the cases.9 Among the patients with ulcers, gastric ulcers were more common than duodenal ulcers (54.4% and 37.1%, respectively). Table 1 presents the main causes of upper gastrointestinal bleeding diagnosed through endoscopic examination.10,11
Peptic ulcer disease |
Esophageal and gastric varices |
Hemorrhagic gastritis |
Esophagitis |
Duodenitis |
Mallory-Weiss tears |
Angiodysplasia |
UGI malignancy |
Anastomotic ulcers |
Dieulafoy (abnormally large tortuous submucosal artery) lesions |
In contrast to these data, other authors advocate that tumorsare the most common cause of upper gastrointestinal bleeding in cancer patients, since it can directly bleed from lesions of the gastrointestinal tract, including esophageal cancer, gastric cancer, gastric lymphoma, gastrointestinal stromal tumors, and metastatic tumors involving the stomach. A retrospective review of patients referred to an endoscopy unit in a reference cancer center in Brazil found that tumorswere the most common cause of bleeding (23.8%), followed by varices (19.7%), peptic ulcer (16.3%), gastroduodenal erosions (10.9%), angioectasia (7.5%), undefined (6.8%), metastasis (4.1%), esophageal mucosal injury (4.1%), hypertensive gastropathy (2.0%), Mallory-Weiss tear (1.4%), and others (3.4%).12 In the same study, consideringonly the patients with luminal tumors in the upper gastrointestinal tract (oropharynx, hypopharynx, esophagus, stomach and gastric stump), the main causes of bleeding were tumors (84.4%), ulcers (6.3%), undefined (6.3%), and varices (3.1%). Among patients with tumors located outside the gastrointestinal tract, the causes of bleeding were similar to those found in the general population (ulcer, gastroduodenal erosions, and varices); nevertheless, metastases were the source of bleeding in a significant number of patients (11%). Although these findings are from one reference center and, for better reliability, the data needs to be reproduced in other studies, these results show that tumor bleeding may well play a major role in the source of acute blood loss, especially in patients with upper gastrointestinal tract cancer.
Clinical history
The medical history, physical exam, and initial laboratory exams are important in the evaluation of recommendations for volemic resuscitation (crystalloids or hemoderivates), endoscopy, and surgery. The initial evaluation must be objective, in search of a diagnosis, so as not to delay the beginning of resuscitation procedures, and a complete medical history must be obtained after the patient has been stabilized.
Past episodes of gastrointestinal bleeding and its causes should be studied, giventhat up to 60% of the cases of upper gastrointestinal bleeding result from a gastrointestinal lesion with prior bleeding, the use of non-steriodal anti-inflammatory drugs (NSAID) or aspirin, anticoagulants, and antiplatelets.13 Involuntary weight loss, dysphagia, smoking, and alcoholism suggest malignity, especially within the gastrointestinal tract. Patient medical history provides the path to the cause of upper gastrointestinal bleeding, as summarized in Table 2.11
Etiology of the bleeding |
Data from clinical history |
Mallory-Weiss lesion |
Alcoholism/hyperemesis prior to hematemesis |
Esophageal ulcer |
Odynophagia, Gastroesophageal Refluz Disease (GERD), intake of esophageal-toxic medication |
Peptic ulcer |
Epigastric pain or in hepatitis D virus (HDV), NSAID |
Acute gastric mucosal lesions |
Patients in intensive care, active acute gastrointestinal bleeding after hospitalization, respiratory insufficiency, or a combination of these |
Vericose veins, hypertensive gastropathy |
Alcoholism, Cirrhosis |
Gastric antral vascular ectasia |
Cirrhosis, renal insufficiency |
Malignity |
Recent weight loss, dysphagia, cachexia, early satiety |
Angiodysplasia |
Renal insufficiency, hereditary hemorrhagic telangiectasia |
Aortoenteric fistula |
Known or already operatedaortic aneurysm |
Table 2 Data obtained through clinical history regarding the cause of gastrointestinal bleeding
The presentation and appearance of blood helps to locate the bleeding and evaluate its severity. Melena is known as black feces with a bad odor. The cases of excessive upper gastrointestinal bleeding appear with melena in nearly 75% of the cases and with hematemesis in approximately 50%.14 Hematemesis indicates bleeding proximal to the Treitz ligament and suggests a greater volume of bleeding when compared to patients that present a dark colored vomit. Approximately 90% of the cases of melena result from upper gastrointestinal bleeding due to the degradation of the blood during gastrointestinal passage and only 10% are secondary to lower gastrointestinal bleeding (hemorrhaging distal to the Treitz ligamento).11 Hematochezia can occur in upper gastrointestinal bleeding, and should be eliminated, when accompanied by signs of hypovolemia or hypoperfusion.
Physical exam
The physical exam should be performed with emphasis on the evaluation of the hemodynamic repercussion, abdominal exam (search for complications, such as: signs of peritoneal irritation), stigmas of portal hypertension (jaundice, vascular spiders, palmar erythema, hepatomegaly, ascites, and caput medusa), and rectal examination. The severity of blood loss is estimated by the hemodynamic state and alterations in target organs (Table 3).11 As regards the abdominal exam, increased hydroaerial noises (HAN) are consistent with upper gastrointestinal bleeding, since blood in the proximal intestine is an irritation that stimulates peristaltic movements, while normal HAN are more consistent with lower gastrointestinal bleeding. Diminished HAN suggest intestinal ischemia, paralytic ileum, or late mechanical obstruction. Abdominal pain is uncommon in the cases of uncomplicated upper gastrointestinal bleeding. Intense abdominal pain suggests gastrointestinal bleeding associated with mesenteric ischemia, intestinal obstruction, or gastrointestinal perforation.11 Peritoneal irritation or involuntary defense demands the exclusion of gastrointestinal perforation before performing the upper gastrointestinal endoscopy (UGE). A careful rectal exam should be performed, including the determination of the type of bleeding (hematochezia or melena), as well as the inspection of external hemorrhoids and anal fissures.
Severity of bleeding |
|||
Physical signs/ degree of volemic loss |
Average |
Moderate |
Severe |
Volemic Loss |
<1L |
1-2L |
>2L |
Arterial Loss |
Normal |
Normal –Slightly below the lower limit |
Hypotensive |
Orthostatic Hypotension |
No |
Possible |
Common |
Tachycardia |
>100 |
>120 |
>140 |
Skin |
Hot, well perfused |
Cutaneous pallor |
Cold and sudoretic |
Respiratory Rate |
20-30 |
30-35 |
>35 |
Urinary Output (Ml/H) |
20-30 |
15-May |
Negligible |
Mental State |
Moderately anxious |
Anxious, confused |
Confused, lethargic |
Table 3 Correlation between the physical signs and the severity of upper gastrointestinal bleeding
Laboratory exams
The reduction of hemoglobina reflects the degree of blood loss after 24 hours or more of acute bleeding, given that, initially, there is a proportional loss of plasma and red blood cells. Later, the reduction of hemoglobin occurs due to the migration of extravascular liquid, with this dilution increased by venous hydration. The erythrogram in series is used to evaluate the severity of upper intestinal bleeding, but it must be associated with the hemodynamic evaluation, since hyperhydration can falsely diminish to hemoglobin. Central venous pressure, or Swann-Ganz catheter, aid in the volemic resuscitation of patients with comorbidities in which the intravascular volume is difficult to evaluate clinically,15,16 such as in patients with cardiac or renal insufficiency. Additional laboratory exams include the coagulogram, electrolyte, arterial gasometry, urea, creatinine, and hepatic enzymes. The application of a basic electrocardiogram in the first stage of medical care is useful, since bleeding and hypovolemia can precipitate myocardial ischemic events.17 With the elderly patient who remains with hypotension and tachycardia, despite the adequate volume replacement, one should contemplate the possibility of an acute myocardial infaction. The initial X-ray of the thorax is also useful in the case of an unfavorable evolution associated with pulmonary aspiration.
Nasogastric catheter
The passage of a nosagastric catheter and gastric irrigation with saline solution is beneficial to detect the presence, aspect, and volume of blood, to clean the field for endoscopic visualization, and to prevent the aspiration of gastric contents. An aspiration with a large quantity of blood, eliminated through a traumatic endotracheal intubation, can confirm upper gastrointestinal bleeding. The bright red blood suggests active bleeding, while the dark red blood suggests recent bleeding. The continuous aspiration of bright red blood suggests severe active bleeding, associated with a higher rate of evidence of active bleeding and other endoscopic stigmas of recent hemorrhaging while performing an emergency UGE, as compared to a dark red aspiration or without the presence of blood.18
An aspiration without blood does not exclude the possibility of recent gastric bleeding that ceased a few hours earlier, due to the previous gastric emptying or duodenal bleeding, whose content did not flow back through the pylorous.19 One bilious aspiration, without blood, practically excludes this alternative; however, it does suggest that the bleeding is distal to the Treitz ligament or that it had ceased many hours earlier. The introduction of the nasogastric catheter is uncomfortable but rarely causes complications. The main complications include nosebleeds, due to the traumatic passage of the tube, and gastric erosions, due to the aspiration of the catheter. The erosions are recognized as being multiple, collinear, equidistant, rounded, and relatively uniform, as well as being in the same stage of evolution.11 In a review of 152 nasogastric catheterisms in patients with upper gastrointestinal bleeding, considering that of these, 125 had suffered an acute myocardial infarction within a period of 30 days prior to the procedure, only two (1.3%) cases presented clinically significant complications, including one case of nosebleeds and another of gastric erosion induced by the tube, both of which were treated with a blood transfusion (red blood cell concentrates), evolving with no clinical sequelae.18 The risk of nosebleeds is reduced with the use of a correct technique, when introduced softly, with lubrification of the end of the tube, with cooperation from the patient, suspension of venous heparine therapy four hours prior to the introduction of the catheter, and avoiding the insertion when a coagulopathy is detected. The risk of introducing gastric erosions is diminished when suction is applied in the nasogastic probe in a intermittent manner, using low-pressure negative aspiration and the early removal of the tube after the bleeding has ceased through the use of flexible probes with multiple orifices.
Risk stratification
Approximately 80% of the bleeding of the upper gastrointestinal tract ceases spontaneously, without recurrence. Morbidity and mortality occur in the remaining 20%, with persistent and recurrent bleeding. The stratificiation of the patients in low and high risk categories for rebleeding and mortality is an essential step toward the drafting of therapeutic proposals.20,21 To achieve this, scales (scores) were drafted from the clinical, laboratory, and endoscopic criteria. The prognostic predictors most commonly used in the evaluation of patients with upper gastrointestinal bleeding are: a) The Blatchford Score22 (Table 4), consisting of only clinical and laboratory parameters, is proposed to predict the need for treatment (blood transfusion, endoscopic hemostasis, or surgical intervention) in patients with upper gastrointestinal bleeding even before performing the UGE. This score also makes it possible to perform the triage of those patients that require an emergency high gastrointestinal endoscopy (in the first 24 hours).
Admission Parameters |
Score |
Urea (Mg/Dl) |
|
>6.5<8.0 |
2 |
>8.0<10.0 |
3 |
>10.0<25.0 |
4 |
>25.0 |
6 |
Hemoglobin (G/Dl) |
|
Man |
|
>12.0<13.0 |
1 |
>10.0<12.0 |
3 |
<10.0 |
6 |
Woman |
|
>10.0<12.0 |
1 |
<10.0 |
6 |
Systolic blood pressure (mmHg) |
|
100 to 109 |
1 |
90 to 99 |
2 |
< 90 |
3 |
Other parameters |
|
Pulse> 100 Bpm |
1 |
Melena as the 1st Symptom |
1 |
Syncope |
2 |
Hepatopathy |
2 |
Cardiac Insufficiency |
2 |
Table 4 Blatchford Score
*A score of above zero can be indicative of the need for treatment (blood transfusion, endoscopic hemostasis, or surgical intervention).
b) Rockall et al.23 based on a study involving 5,810 patients, drafted a standardized score for the evaluation of factors that predict the mortality and risk of rebleeding in patients with upper gastrointestinal bleeding (Table 5). The risk factors observed were age, the presence of a state of shock, existence of comorbidities, endoscopic diagnosis, and endoscopic stigmas of recent bleeding. According to this study, 41.1% of the patients who had 08 (eight) or more points in this score died due to upper gastrointestinal bleeding, and 53.1% presented re-bleeding. By contrast, among those patients that presented a score of less than or equal to 2, less than 1% of the cases died and less than 6% presented rebleeding.
Score |
||||
0 |
1 |
2 |
3 |
|
Age |
<60a |
60 a 79a |
>80a |
|
State of Shock |
Pulse<100 Sist BP>100 |
Pulse>100 Sist BP<100 |
Pulse>100 Sist BP<100 |
|
Comorbidities |
Absent |
Circulatory or coronary insufficiency |
Renal or hepatic insufficiency or disseminated malignant disease |
|
Endoscopic Signs of Bleeding |
None/flat and dark coagulation |
Raised, bright blood clot or visible blood vessel |
||
Diagnosis |
Mallory Weiss syndrome/absence of other diagnosis |
All other diagnoses |
Malignant disease of the upper gastrointestinal tract |
|
Table 5 Rockall Score
The use of a risk stratification score has the objective of providing the emergency ward doctor with criteria for the proper triage of patients according to the severity of their medical condition. The Rockall or Blatchford scores are especially useful to define those patients with a low risk of morbi-mortality, who can be released from the hospital earlier, without the need for intensive therapy and without the need for emergency UGE in the first 24 hours. The use of these scores is therefore recommended as a basis for risk stratification in these urgency and emergency units. A score of above 02 can be indicative of patients at risk of rebleeding or death.
Initial approach
The initial stabilization must include the evaluation of the airways, respiration and circulation, venous access, and the collection of laboratory exams, in addition to, when indicated, the administration of fluids, blood transfusion, cardiorespiratory support, and treatment of severe concomitant diseases, such as sepsis or acute myocardial infactions. In patients that present respiratory insufficiency or hemodynamic instability, the UGE should be delayed until the patient is adequately resuscitated and stabilized.
General support measures
Generally, patients receive supplementary oxygen by nasal catheter to compensate for the loss of the carrier capacity of oxygen, due to the loss of red blood cells. In the presence of massive bleeding, persistent hematemesis, hypoxia, tachypnea, or alteration of one’s mental state, an immediate and definitive airway must be evaluated (example: endotracheal intubation) to protect the aspiration airways and supplementary oxygenation.24 Non-invasive monitoring with oximetry, non-invasive measuring of blood pressure, and continuous ECG are recommended. In patients with hemodynamic instability, it may be necessary to apply an invasive monitoring with central venous pressure (CVP), intra-arterial pressure (IAP), or Swan-Ganz catheter. A vesical probe with a Foley catheter is indicated in patients in a state of shock, or in those that present massive bleeding, in order to control urinary output.25
Patients are evaluated to determine the severity of bleeding (Table 3) and define the requirements for crystalloid infusion (saline solution or lactated ringer’s solution) or a blood transfursion (red blood cell concentrates, fresh frozen plasma, or platelets), which should be properly washed due to the presence of concomitant diseases, especially cardiovascular disease and renal insufficiency. Intravenous access is guaranteed by two thick caliber peripheral venous catheters. Thepatients with active bleeding can require around two to three liters of crystalloid solution to maintain the blood pressure, and if this does not produce a response to the initial rapid blood infusion, the transfusion of a specific type of blood may be necessary.
The transfusion of red blood cell concentrates serves to improve the oxygenation of the tissues and impede damage to the target organs. The need for blood transfusions is individual, with no level of hemoglobin presenting an absolute indication, with the requirements determined by various factors, including age, presence of comorbidities, cardiovascular state, basal hemoglobin, and volume of blood, together with the current level of hemoglobin. Red blood cell concentrates should be transfused in patients who present an excessive blood loss, who present persistent active bleeding, and who present signs of cardiac, renal, or cerebral hypoperfusion.5,11 Patients who present hemodynamic instability associated with active bleeding or recent engina are recommended for early blood transfusion. Patients with vericose upper gastrointestinal bleeding should be treated conservatively, transfused with a hemoglobin rate of less than 7.0 g/dl to avoid a worsening of bleeding caused by an increase in rebound portal hypertension.11
Young and healthy patients who do not present uncompensated comorbidities more adequately toleratehemoglobin rates of 7.0 g/dl, while the elderly, as well as cardiopathy and chronic renal patients, have a lower cardiopulmonary reserve and are unable to tolerate hemoglobin of less than 10 g/dl.20 The coagulopathy can exacerbate the bleeding and should be treated with a transfusion of fresh frozen plasma or platelets, on a case by case basis. One useful piece of advice is to transfuse 01 unit of fresh frozen plasma for each 04 units of red blood cell concentrate in order to substitute the coagulation factors that have been lost.25 The international normalized ratio (INR) of less than 1.5 does not require treatment. Discrete thrombocytopenia (50,000 - 90,000 platelets/ml) rarely contributes to upper gastrointestinal bleeding, whereas a count of less than 50,000/ml in the presence of active bleeding can require a transfusion of platelets. This general rule is individualized according to multiple factors, including the severity of bleeding, the presence of other coagulopathies, and an altered platelet function, such as those induced by NSAID.26 When surgery is deemed necessary, the coagulopathies should be corrected before beginning the procedure.
Blood transfusions rarely have collateral effects; however, if they occur, they can be severe. Despite the triage performed with blood donors, the HIV virus, type 1 and type 2 HTLV (Human T-cell Lymphotropic Virus), hepatitis B, hepatitis C, and parvovirosis, though rare, can be transmitted through blood transfusions. In addition to bacterial infections, particularly the Yersinia enterocolitica in the transfusion of red blood cell concentrates and Staphylococcus aureus in the transfusion of platelets.26 A rapid transfusion can induce congestive heart failure and pulmonary edema in patients that have suffered prior congestive heart failure or other heart diseases and should therefore be transfused carefully and slowly. Diuretics can be administered both before and during the procedure in these patients.
Clinical treatment
The use of proton-pump inhibitors (PPIs), in cases of upper gastrointestinal bleeding caused by peptic ulcers, significantly reduces the rates of rebleeding, the need for surgical intervention, and the need for endoscopic retreatment, when compared to the placebo or H2 blockers.20,21 In addition, reductions in the mortality rates may well result from the use of PPIs in high-risk patients (active bleeding or non-bleeding visible blood vessels upon application of UGE).27 The venous formulations of PPIs can be administered in bolus or by prolonged infusion and, in the eventual lack of these formulations; the use of doubled oral doses of PPIs (every 12 hours) presents satisfactory results. In patients that, when undergoing an endoscopy, do not present active bleeding, ulcers with visible blood vessels or adhered coagulation (i.e., low risk for rebleeding), the treatment can be started with the oral administration of PPIs. It is suggested that omeprazole be used at a dose of 80 mg, intravenously, in bolus, followed by an infusion of 8 mg/h, for 72 hours, at which time it should be exchanged for 20 mg, administered orally (once per day) for eight weeks.5,27 The suspension of the medication after this period will depend on the correction of the percipient factors, such as H. pylori, NSAIDs, and acetylsalicylic acid (ASA).
The use of other drugs, such as somatostatin or octreotide, can be beneficial due to the effects produced in the reduction of splanchnic blood flow, the inhibition of acid secretion, and the supposed gastric cytoprotective action.27 However, due to the high cost and low availability, the use of these drugs is reserved for rare occasions in which the conventional therapy has proven to be inefficient.
Endoscopic therapy
Over the last decade, many randomized clinical studies, as well as meta-analyses, demonstrated that endoscopic therapy reduces the indices of the recurrence of gastrointestinal bleeding, the need for emergency surgery, and mortality in patients with peptic ulcers with active bleeding or with visible blood vessels.20,21,27 The endoscopic therapy, in the majority of case series, demonstrates a success rate of more than 94% in cases following the initial approach to bleeding lesions. However, the inidices of recurrent bleeding are significant (15-20%), particularly in patients with large and deep ulcers, coagulopathies, conditions of severe comorbidity, hypertension, or bleeding that develops during hospitalization. These are frequently elderly patients with a high risk of surgical mortality and with indices of post-operative mortality of 15% to 25%. One prospective study, carried out with 92 patients with an average age of 65 years who had presented rebleeding after endoscopic control, demonstrated that endoscopic re-treatment reduces the need for surgery, wihout increasing the risk of death and with less complications than surgery.20,28 In patients with persistent hypotension, in which the hemostasis was not successful, repeated and failed endoscopic procedures can negatively affect survival rates. In some studies, early elective surgery, after the endoscopic control of bleeding in high-risk patients, has been reported to be a successful measure to reduce recurrent bleeding, morbidity, and mortality.27 However, this strategy is not universally accepted and requires further study. The combination of the injection of an epinephrine solution and thermal therapies are considered the best approach to control continuous bleeding or prevent rebleeding in visible blood bessels of ulcered lesions.
Currently, in bleeding esophageal varices, the elastic ligature has been preferred as the therapeutic modality. This procedure reduces the risk of rebleeding and appears to improve survival rates. Its efficacy in the short term of schlerotherapy is less than that of elastic ligature. Schlerotherapy is associated with the highest indices of complication, such as perforation and rebleeding. By contrast, the late rebleeding after the elimination of varices though shlerotherpay appear to be less than those from elastic ligature.27,29 Enscopic interventions can be used for the treatment of upper gastrointestinal bleeding related to neoplasias of the gastrointestinal tract. Akhtar et al.30 reported, in a series of 48 consecutive patients with cancer of the esophagogastric junction who were submitted to an endoscopy with coagulation in an argon plasma beam, bleeding was well-controlled in three of the five patients with acute upper gastrointestinal bleeding. However, in patients with excessive bleeding secondary to advanced malignant gastroduodenal neoplasms, endoscopic therapy appears to provide a limited benefit. Loftus et al.31 in a study carried out with 1,083 consecutive patients with upper gastrointestinal bleeding, observed that in 21 patients (1.9%) advanced tumors were detected in the stomach and duodeno, of which 15 received endoscopic therapy, with 11 gastric and 4 duodenal. The endoscopic treatment consisted of an injection of adreline, electrocoagulation, heater probes, argon plasma, sodium tetradecyl sulfate, and laser therapy. The initial endoscopy reached hemostasis in 10 to 15 patients (67%); however, recurrence of bleeding was observed in 8 to 10 (80%), and in all of the 5 in which endoscopic hemostasis was not achieved, bleeding persisted. Five severe complications related to the procedure occurred, of which two were fatal.
Upper gastrointestinal bleeding may sometimes be the immediate cause of death in patients with advanced cancer. Bleeding can result from local blood vessel damage and invasion or from systemic processes (liver failure, radiotherapy, chemotherapy, disseminated intravascular coagulopathy, among others).1 Although most cases of upper gastrointestinal bleeding cease on their own or are controlled endoscopically, some cases appear in which the source of bleeding cannot be indentified or treated endoscopically. If bleeding is heavy (>1.0 ml/min) angiography can be used both to aid in diagnosing the location of the bleeding and to provide angiographic intervention to control the bleeding source. Endoscopy represents a fast, safe, and a minimally invasive option to surgery in selective cases. Because ongoing bleeding typically coincides with the clinical deterioration of the oncological patient, embolization should be performed early in patients with upper gastrointestinal bleeding who have not been treated endoscopically.
Agents used for embolization
Selective embolization of the bleeding artery can be performed with a variety of agents that mechanically occlude the vascular supply of the bleeding lesion (coils, PVA, gelatin sponge), infusion of vascoconstricting agents to decrease the blood flow to the bleeding site, or by a combination of these techniques.2 The agents are either temporary or permanent32 (Table 6). Advantages of using gelatin sponges include cost effectiveness, widespread availability, and temporary occlusion. These sponges are more commonly used in combination with coils and particles when bleeding occurs from a lesion that is not expected to heal spontaneously, such as tumors.33 Coils and microcoils have become the preferred agent for embolizing upper gastrointestinal bleeding by most interventionalists. These can be deployed selectively by means of a microcatheter into the distal bleeding artery, thus preserving the collateral blood supply to other tissues. Vasopressin causes generalized vasoconstriction in vessel walls (arterioles, capillaries, and venules), in turn producing a rapid reduction in the local blood flow which allows for a stable clot formation at the bleeding site. This should be used cautiously in patients with severe hypertension, coronary artery disease, peripheral artery disease, arrhythmias, and congestive heart failure. Rebleeding is common once the vasopressin has been stopped, and its use becomes much less frequent after the introduction of microcatheters and microcoils.34
Agents |
|
Permanent |
Temporary |
- Coils |
- Absorbable Gelatin Sponge (Gelfoan) |
- Glue |
- Vasopressin* |
- Particles (Pva) |
|
- Ethylenevinyl Alcohol Copolymer |
|
- N-Butyl2cyanoacrylate (Nbca) |
|
Table 6 Materials used for embolization
*Vasoconstricting agent.
Technique
Transcutaneous arterial embolization may be useful in selected patients with cancer and upper gastrointestinal bleeding. This procedure is often performed through a femoral approach, but it can also be performed through a brachial or radial approach. It is generally well tolerated under local anesthesia and mild sugetion.35 Transcatheter interventions include selective embolization of the feeding artery (usually the gastroduodenal artery or left gastric artery), “sandwich” coil occlusion of the gastroduodenal artery (coils and sponge), and empiric embolization of the supposed bleeding vessel. In the last case, the placement of clips at the bleeding site during endoscopy helps the interventional procedure.36 Embolization works by decreasing the blood flow enough to achieve hemostasis while maintaining collateral perfusion in other tissues adjacent to the bleeding site. The procedure begins with the selective catheterization of the celiac artery or the superior mesenteric artery, and contrast extravasation into the bowel lumen is considered definitive evidence of a bleeding site. Sometimes there is only indirect evidence of bleeding in an angiography (early draining vessels, neovascularity, arteriovenous fistulas, and the filling of spaces outside the bowel). Treatment with empiric embolization can be performed in these cases (embolization of a vessel thought to be supplying the bleeding source without bleeding having been demonstrated in the angiography).Empiric embolization is as effective as in those patients who show contrast extravasation during angiography.36
Embolization of upper gastrointestinal bleeding in oncological patients is commonly performed with a combination of agents: gelatin sponge (Gelfoan), coils, microcoils, and particles. These embolic agents function similarly to surgical ligation by decreasing perfusion pressure to the bleeding site; the reduction of blood flow promotes clot formation. Due to the dual blood supply of the stomach and duodenum, the risk of bowel ischemia is minimal when embolization is performed with coils and absorbable gelatin sponges. The catheter or microcatheter should be inserted into the selected artery in order to avoid deployment of embolic materials in undesired areas. This technique prevents ischemia or infarction, common complications of the procedure (Figure 1 & 2).
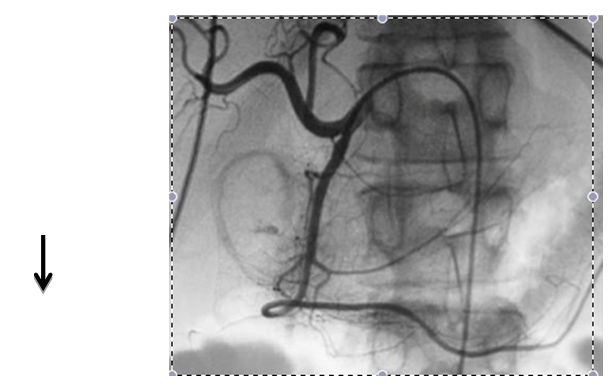
Figure 1 Embolization of the gastroduodenal artery in a patient with upper gastrointestinal bleeding. Common hepatic arteriogram shows a focus of extravasation in the region of the duodenum (arrow).
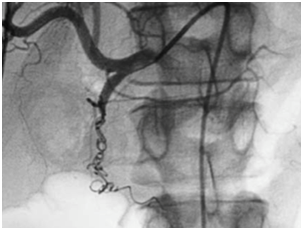
Figure 2 Common hepatic arteriogram after embolization with coils and gelatin sponge shows occlusion of the gastroduodenal artery.
It is imperative to occlude the gastroduodenal artery proximal and distal to the bleeding site. If only the proximal portion of the gastroduodenal artery is occluded, bleeding may persist through the pancreaticoduodenal arcade supplied by the superior mesenteric artery (collateral bleeding through the “back door”). Occasionally, it may be necessary to embolize these arteries in a superselective manner through the superior mesenteric artery using coaxial microcatheters. Embolization is usually most effective when performed in combination with medical therapy to correct the underlying etiology of bleeding. If the patient is unable to form a clot, embolotherapy is likely to fail due to the abundant collateral arterial supply to the stomach and duodenum.
Complications
Embolization is restricted to areas where the blood vessels are accessible by the catheter and where embolization of the blood vessel does not result in the ischemia of key organs.37 In a review that included patients undergoing embolization for upper gastrointestinal bleeding, complications occurred in 5% to 9% of the patients (bowel ischemia and infarction accounted for the majority of complications)38 (Table 7).
Complications |
- Bowel Infarction |
- Groin Hematoma* |
- Pseudoaneurysm* |
- Dissection* |
- Embolism* |
- Thrombosis* |
- Renal Failure* |
Table 7 The complications of embolization
*Complications associated with arteriography itself.
Efficacy of embolization
Rebleeding is common with intra-arterial vasopressin (9-56% depending upon the bleeding site) and less common with other embolization agents.39,40 Efficacy of embolization to control upper gastrointestinal bleeding in oncological patients ranges from 60-75% and varies with the type of lesion.41 If embolotheraphy is unsuccessful, even after more than one procedure, then open surgery may be an alternative to treat these patients (Table 8).
Clinical Failure |
- Corticosteroid use |
- Anticoagulant use |
- Coagulopathy |
- Multiorgan failure |
- Previous surgery |
- Embolization with coils alone |
- Longer Time for angiography |
Table 8 Factors associated with failed embolization.42
Despite The advances in endoscopic and phramacological therapies, 10% to 15% of the patients presented rebleeding, with the mortality rates in these cases being four to five times greater.43 The recommendations of surgical treatment for upper gastrointestinal bleeding are summarized in Table 9. The type of surgical treatment depends on the cause of the upper gastrointestinal bleeding, hemodynamic repercussion, comorbidities, complications (perforation), availability of material, and technical knowledge (minimally invasive approach). Czymek et al.44 in a study carried out with 91 patients (58 men, 33 women) who had been admitted between 2000 and 2009 at the College of Medicine of the University of Luebeck (Germany) and who had undergone surgical treatment due to upper gastrointestinal bleeding, the causes of bleeding included duodenal ulcers in 57 patients (62.6%), gastric ulcers in 25 (27.5%), gastric and duodenal ulvers in 7 (7.7%), and malignant neoplasms in 2 (2.2%). The surgical treatment consisted of ulceroraphy (52.7%), partial gastrectomy with Billroth II reconstruction (31.9%), partial gastrectomy with Billroth I reconstruction (4.4%), gastric wedge resection (4.4%), and total gastrectomy (3.3%). Jairath et al.32 in a natonal auditing in the United Kingdom, carried out with 4,478 patients in 212 institutions, 97 patients underwent surgical treatment. The surgical procedure performed included ulceroraphy (69%), ulcer excision (3%), ulcer excision with vagatomy/pyeloplasty (2%), partial gastrectomy (9%), and others (16%). The recommendations for surgery were uncontrollable bleeding (82.5%), peritonitis/perforation (6.8%), malignity (4.9%), and others (8.7%). Patients with cancer can benefit from early surgical procedures to control bleeding, especially if endoscopic treatment is not possible. Despite the improvement in the mortality rates with surgery, curative ressecton is relatively uncommon and post-operative complications are common.5
- Hemodynamic instability after vigorous treatment (>06 units of transfusion) |
- Failure of endoscopic treatment |
- Recurrence of bleeding after two attempts of endoscopic treatment |
- State of shock associated with recurring bleeding |
- Continous bleeding with the need for the transfusion of 03 units/day |
Table 9 Indication of surgery in acute upper gastrointestinal bleeding
The main causes of upper gastrointestinal bleeding are similar between the oncologic patients and the population in gereral. The minority of theseare due to tumor bleeding, and the aplication of radiotherapy is restricted to these cases.45 Acute tumor bleeding typically represents a late stage of disease when the neoplasm outgrows its blood supply and causes mucosal ulceration. Virtually any tumor type may bleed. Patients with severe bleeding resulting from malignant upper gastrointestinal tumors show a poor prognosis, the majority of whom die within 12 months.46 Radiotherapy in these cases is most often palliative. The radiotherapy treatment approach depends on the performance status scale, the previous treatment submitted by the patient, his or her hemodynamic stability, among other factors. In the patients scheduled receive radiotherapy, it is important to monitor hemoglobin levels, asit is known that the presence or absence of molecular oxygen dramatically influences the biological effect of X-rays. A review of relevant clinical data suggests that a maximum oxygenation status in solid tumors is to be expected within the range of 12 g/dl <cHb <14 g/dl for women and 13 g/dl <cHb <15 g/dl for men; however, these values are dificult to achieve in patients with upper gastrointestinal bleeding.47 To produce this effect, molecular oxygen must be present during the radiation exposure or at least during the lifetime of the free radicals generated by the radiation. Oxygen makes the damage produced by free radicals permanent, and hypoxia may play an important role in malignant progression.48 The esophageal, gastric, and hepatic tumours are examples of tumors that can be treated with radiotherapy in these situations.
Bleeding gastric cancer responses to radiotherapy are not as immediate as with palliative surgery.49 External beam radiation therapy plays a well-defined role in the control of bleeding in patients with localized but unresectable tumors.50–55 Palliative doses ranging from 8 Gy per single fraction to 40 Gy in 16 fractions can control the bleeding in approximately 80% of all patients.55 Treatment is well tolerated with low toxicity and can last the duration of most patients’ lives. There was no difference in response between low and high biologically effective dose regimes. Chemoradiotherapy have also demonstrated a durable effect.49 Bleeding from hepatic tumors directly invading the gastrointestinal tract is uncommon but should be considered. The treatment of choice against the direct invasion of the gastrointestinal tract by hepatic tumors is palliative surgical resection; however, few patients can receive this procedure due toits poor performance status. Palliative radiotherapy may offer an alternative choice if the patient is not a candidate for surgical ressection. The bleeding can be stopped by doses such as 50 Gy. Doses similar to these are apparently well tolerated by patients who present no liver cirrhosis and by those who had Child’s class A liver cirrhosis.56
Esophageal cancer may also bleed. External beam radiotherapy with concurrent chemotherapy is a standard approach for patients with locally advanced unresectable or inoperable thoracic and abdominal esophageal cancer who are medically able to tolerate chemotherapy. The landmark RTOG 85-01 trial, demonstrated that the addition of concurrent chemotherapy to conventional fractionation radiotherapy provided a significant survival benefit when compared to treatment with radiotherapy alone.57,58 In this trial, the radiotherapy dose used was 50 Gy in 25 fractions over five weeks. In patients who cannot tolerate chemotherapy, palliation radiotherapy can be performed in hypofractionated schemes. The minority of esophageal cancers arise in the cervical portion of the esophagus, the majority of which are locally advanced at the time of diagnosis and may bleed.59,60 The treatment of choice in these tumors is radiotherapy combined with concurrent chemotherapy rather than surgery. It is also important to note that bleeding can occur as sequelae of radiotherapy treatment due to radiation induced ulceretion or telangiectasia. Thrombocytopenia is common in patients undergoing chemotherapy together with radiotherapy, which also favors bleeding. Argon plasma coagulation, hyperbaric oxygen therapy, and formalin therapy are treatment choices in these cases.61
In patients with upper gastrointestinal bleeding secondary to direct tumor effect, in addition to clinical support aimed at stabilization, patients frequently receive one therapeutic modality, depending on the situation (endoscopic intervention, radiotherapy, or endovascular device). Treating the primary cause of the bleeding (primary cancer or an invading metastasis) with chemotherapy or other systemic agents (ex. monoclonal antibodies) can also be useful, but it is most commonly performed after inicial stabilization. In patients with bleeding gastric lymphomas, chemotherapy regimens (R-CHOP in diffuse large B-cell lymphomas, for example) can achieve high response rates and contribute to hthe cessation of acute blood loss. Corticosteroids can play a special role in this specific scenario. Each case must be fully individualized, and the best approach should be chosen on a multidiscliplinary basis.
Acute upper gastrointestinal bleeding has a relatively high rate of mortality, despite the advances in diagnoses and treatments. Malignant neoplasms, though the least likely primary cause of gastrointestinal bleeding, and hematologic and anatomic changes related to cancer, hinder its management. Moreover, oncologic treatments can prsent a wide range of consequences that can lead to occult or massive hemorrhaging. The initial approach to gastrointestinal bleeding is similar in patients with or without cancer; nevertheless, special attention should be given to this condition due to the complication factors, which include hematologic and metabolic diseases, as well as structural abnormalities.
Minas Gerais Research Support Foundation (FAPEMIG) and National Council for Scientific and Technological Research (CNPq) for their financial support.
None.
The author declares there is no conflict of interest.
None.

©2016 Alberti, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.