eISSN: 2373-6372


Mini Review Volume 11 Issue 3
1Department of Endoscopy, University of Guadalajara, México
2Department of Gastroenterology, University of Guadalajara, México
3Department of Coloproctology, University of Guadalajara. Hospital Civil of Guadalajara, México
4Department of Gastroenterology, IMSS, México
5Medicine School of Medicine, Universidad Autónoma de Guadalajara, México
6Hospital Civil of Guadalajara, México
7Department of Health Public, University of Guadalajara, México
Correspondence: Yolanda Cortés Aguilar, Endoscopia Gastrointestinal Videoendoscopia Américas, niversidad de Guadalajara. Av. Américas 226-1 Col. Ladrón de Guevara, Guadalajara, Jal, México, Tel 33 1918 0101
Received: May 02, 2020 | Published: May 18, 2020
Citation: Cortés AY, Miguel JLA, Valenzuela J, et al. Update on mechanisms of transmission of severe acute respiratory syndrome Coronavirus (SARS-COV-2) in endoscopy. Gastroenterol Hepatol Open Access. 2020;11(3):112-116. DOI: 10.15406/ghoa.2020.11.00424
Purpose: To update the mechanisms of transmission of infection of severe acute respiratory syndrome coronavirus (SARS-COV-2) during endoscopy, virus is transmitted via multiple routes the spread of infections such as SARS-COV-2 in endoscopic procedures occurs in asymptomatic patients or for emergency procedures. All endoscopies should be considered aerosol-generating procedures in the outbreak. The virus is transmitted through direct contact with multi-organ secretions days before and after respiratory distress syndrome by people, droplets, aerosols, endoscopes shows potential risk to infection to staff and patients. Knowledge of the mechanisms and diagnosis will allow reduce of transmission during endoscopic procedures and implement strict follow-up of recommendations for SARS-COV-2 prevention. The principal pathophysiologic mechanism to infect tissues is the expression of angiotensin-converting enzyme II receptor (ACE-2) the characteristic of the virus to enter the human cell is expressed in the lungs, gastrointestinal (ileum, colon, liver), ocular, blood, stool, bone marrow (73.3% vs.14.3%, p.0.033) vs. patients with respiratory symptoms so transmission can be done through a high or low endoscopic procedure.
Aim: To review an update on mechanisms of transmission another than respiratory.
Methods: Based on recent literature review we summarize from available published of different mechanisms of transmission and pathogenicity of SARS-COV-2 that putting in risk of transmission during endoscopy procedures. Criteria used were PubMed for articles describing gastrointestinal endoscopy-associated infections by aerosols, fomites and outbreaks published from 2019 to 2020.
Results: Aerosols and fomites are important for transmission; the viral tropism and life cycle are their principal mechanism with the receptor ACE-2 in tissues.
Conclusions: Results of this review show that virus can be transmitted through direct contact
with secretions and fomites days before and after respiratory distress syndrome with infection in multiple organs in patients with SARS-COV-2.
Keywords: transmission, mechanisms, endoscopy, aerosols, SARS-COV-2
Coronavirus disease 2019 (COVID-19) has declared pandemic by WHO. The virus has spread to over 100 countries, infecting more than 700,000 people with 35,000 deaths globally. Non-respiratory symptoms can also present and are route for transmission and spread for SARS-COV-2.1 The prevalence of extra-pulmonary symptoms at risk of transmission has been found are: bone marrow, ileum, colon, liver, kidney, skin, ocular, oropharyngeal secretions.2 The virus highly contagious, has quickly spread throughout the world with progression to acute respiratory distress syndrome and death.3. Is associated with ACE-2 expressing cells in these tissues to infect cells.4
All endoscopies should be considered aerosol-generating procedures,5 the transmission could be done by aerosols and fomites, the virus is infectious for hours depending of the inoculum shed and the virus receptors so knowing the mechanism associated with spread helps to reduce infection.6
Risk for transmission to endoscopy personnel during gastrointestinal endoscopy procedures. The virus and the endoscopy unit are route for risk of infection by SARS-COV-2 from inhalation of aerosols (airborne droplets), fomites, conjunctival contact, and feces and touch contamination with high risk because close physical distance and direct transmission.7–9
Coronaviruses are unsegmented viruses, with six species that cause infection in humans, mostly with mild respiratory symptoms, they belong to the family Corona-viridae, order of Nidovirals (Alpha, Beta, Gamma and Delta-coronaviruses). Beta-coronavirus is similar to a crown with spikes on the outer surface, small diameter (65-125 nm), the nucleic material contains a single chain, (length 26-32kbs). Coronavirus exists among humans and animals closely related to human beings, (bats, mice, cats, dogs, pigs, and cattle). The genomic analysis of SARS-COV-2 is phylogenetically related to bat, civet and camel viruses and possible primary reservoir with evidence of the virus for more than 30 years, the spread through nosocomial infection to health personnel and patients is highly transmittable with rapid human to human transfer.10
Mechanism of pathogenicity
Mechanisms are related with viral load and their capacity to infect cells by viral replication through many routes. Virus needs a co-receptor and auxiliary membrane proteins to infect a tissue, viral particles recognize host receptors via spike glycoprotein, enter host cells and replicate.11,12
The meta-analysis by Fu et al. found prevalence of 16 clinical symptoms among SARS-COV-2 patients, fever (83.3% [95% CI 78.4–87.7]), cough (60.3% [54.2– 66.3]), and fatigue (38.0% [29.8–46.5]) with highest prevalence hypertension (17±7, 95% CI 14-22%), diabetes (8±6, 95% CI 6-11%), cardiovascular (5±4, 95% CI 4 -7%), respiratory (2±0.95% CI 1-3%). When compared to non-severe patients, the OR for hypertension, cardiovascular, respiratory disease in severe patients was (OR 2.36, 95% CI: 1.46-3.83), (OR 2.46, 95% CI: 1.76-3.44) and (OR 3.42, 95% CI:1.88-6.22) respectively. The origin and transmission to humans is not known15 observations indicate that many patients present initially with diarrhea, conjunctivitis, anorexia, vomiting, abnormal hepatic function tests not necessarily with respiratory symptoms at first.16–18
Real time- PCR is sensitive and reliable, viral loads, measure by quantitative real-time analysis with RT-PCR in smears and culture19 found a sensitivity 66.7% specificity 95%, rate of 2.9 copies per reaction (seven days since onset).20,21 Real-time PCR and smear diagnostics yielded an odds ratio OR=1.91, 95% confidence interval (CI)=1.51-2.41, Z=5.43, P<0.5, while the combination of real-time PCR and culture yielded OR=2.44, 95%CI =1.77-3.37, Z=5.41, P<0.05.22
Viral load of different tissue samples were positive according specimen types tested: n=27 (14 positive nasopharyngeal swabs), 9 throat swabs, 3 positive sputum, two positive nasal swab, one nasopharyngeal aspirate and one a positive endotracheal aspirate,23,24 Yu et al, found in nasal swabs 16.4% (9/55), throat swabs 37.3% (50/134), and sputum 66.4% (77/116). The positive rate of sputum samples was significantly higher than throat and nasal swabs. The average viral load in sputum (17429±6920 copies) was significantly higher than in throat swabs (2552±1965 copies, p<0.001) and nasal swabs (651±501 copies, p<0.001).25 Incubation: 5.5days average (x=0-14).
The results showed that the viral load in the early and progressive stages were significantly higher than that in the recovery stage (46800±17272 vs 1252 ± 1027, p<0.001). Antibody: The dynamics of total antibody IgM and IgG against SARS-COV-2 in blood samples (173) cases were first detected, followed by IgM and IgG (increased during the first two weeks), seropositive 50% on the 11th-day and 100% on the 39th-day. The seroconversion of Antibody was significantly quicker than IgM (p=0.012) and IgG (p<0.001). At seven days since onset, the antibody had a positive rate (38.3%), the antibody of RT-PCR since day 8 after onset, reached 90% across day 12 after onset.26
Transmission by aerosols.Aerosols, superfice stability and half live of SARSCOV-2 were studied and applied to copper, cardboard, stainless steel, and plastic (at 21 to 23 °C,40% humidity 7days).27
Asymptomatic disease, or mild disease occurs in 80% of patients, the viral load detected in asymptomatic and symptomatic patients is similar according the findings of Zhu et al.29 with presence of live viral loads in the blood, mouth and nose, being higher in the nostrils of patients with severe disease compared to patients with mild/moderate symptoms so the potentially infected biological samples during endoscopy is through the face. The asymptomatic SARS-CoV-2 can be positive in the stool samples but negative in nasopharyngeal swabs for a long time and the viral load in nasopharyngeal secretions is present before the onset and serum antibody with easy transmission.30–33
Values of viral load in nasal and throat samples from severe symptoms and asymptomatic patients show importance in the first three days of the infection,27 the cycle threshold (Ct) values corresponded to the viral load Table 1 & Figure 1.
|
|
Cycle threshold (Ct) value |
Viral load |
|
First day |
30.76 |
1.5x104 |
|
Second day |
27.67 |
1.5x105 |
|
Third day |
24.56 |
1.5x106 |
|
Forth day |
21.48 |
1.5x107 |
Table 1 Viral load in nose and throat in 17 symptomatic patients in relation to the day of appearance of symptoms
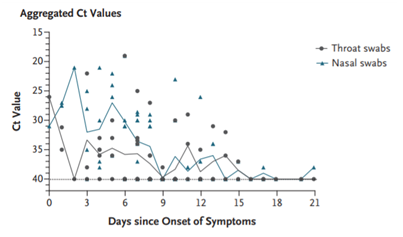
Figure 1 Viral load in nose and throat in 17 symptomatic patients in relation to the day of appearance of symptoms.
Saliva
Transmission mechanism: Live viruses infect epithelial cells in salivary glands (91.7%), saliva contaminates by coughing and drops from the respiratory tract and during normal breathing, To KK et al.,34 analized real-time-PCR. The mean of sample was 2 days (range, 0-7days). The average viral load in the first specimen was 3.3x106 copies/mL (range, 9.9 x102-1.2x108 copies/mL), the highest viral load (83.3%) (Figure 2). Patient H had a higher viral load on the first day of hospitalization (6.8x107copies/mL) than on the day of admission (5.7×107 copies/mL) patient B, the virus in saliva was still detected on day 11 after hospitalization.
Sputum
Transmission mechanism: By direct contact, aerosolized liquids of the patient or infected healthcare workers with greatest risk related with distance, a range of one meter may protect.35 Yu et al.25 found an average viral load in sputum (17429±6920 copies) higher than in throat swabs (2552±1965 copies, p<0.001) and nasal swabs (651±501 copies, p<0.001).36
Eyes
Transmission mechanism: By contact with the face and eyes during the procedures and reusable eye protection equipment the ocular surface and nasal cavity is in close continuity for the mucous membrane from puncta into the nasolacrimal duct and nasopharyngeal space. The virus is delivered into the lungs and the gastrointestinal tract. The ACE-2 is present in aqueous humor, conjunctiva and cornea.37
Blood
Transmission mechanism: Low respiratory concentrations of RNA in acute phase have been found with a high viral load of 190copies/mL in plasma, RNA was detectable (45.5%) of samples at day 15-39, the viral load of RNA in plasma/serum in patients with SARS-COV-2, (first 2-3days of onset), were detected with sensitivities of antibodies, IgM and IgG (100.0%, 94.3% and 79.8%, respectively). SARS-COV-2 infect lymphocytes (target for virus), replicate in them with high concentrations of donor lymphocytes (peripheral blood stem cells, bone marrow, granulocyte concentrates) 38 with possible risk of transmission by transfusion.
Gastrointestinal
Patients with digestive symptoms and fever (62%) presented later than those with respiratory symptoms (16.0±7.7 vs.11.6±5.1 days, p<0.001).39 The diarrhea lasted from 1 to 14days, (average 5.4±3.1 days, frequency of 4.3±2.2 bowel movements/day), (16.0±7.7 vs. 11.6±5.1 days, p<0.001). The virus uses the angiotensin II converting enzyme receptor (ACE-2) to enter the human cell and modulate viral replication, by fusion between the virus and the cell membrane and express itself at the viral receptor, could be found viral nucleic acid in stool (53.4%). Patients with digestive symptoms had a longer duration between symptom onset and viral clearance (p<0.001) (fecal virus positive 73.3% vs. 14.3%, p=0.033) vs. those with respiratory symptoms.40
The virus changes the intestinal flora (28%) through immune regulation (inflammatory response and viremia) with abnormal mucosal immune system41 the disorders of the intestinal flora (gut-lung-axis) and digestive symptoms (nausea, vomiting and diarrhea affect the respiratory tract.42 Patients without respiratory symptoms, diarrhea lasted from 1-14 days, (average 5.4±3.1 days, frequency of 4.3±2.2 bowel movements/day), (16.0±7.7 vs. 11.6±5.1 days, p<0.001).43 Patients with diarrhea/nausea/vomiting, were significantly more likely to test positive for SARS-COV-2, than to test negative (61% vs. 39%, p=0.04).44 SARS-COV-2, lead to direct damage to the intrahepatic bile ducts, with ACE2 expression in cholangiocytes (59.7%) and 2.6% in hepatocytes.45
Ocular
Acute conjunctivitis, retinal vasculitis and degeneration with blood-retinal barrier breakdown had positive results for SARS-COV-2, 13 days after onset infection tested positive, (5.2%) (91.7%; 95% CI, 61.5-99.8), virus is detected in conjunctival swabs (RT-PCR). The 31.6%; (95% CI, 17.5-48.7) of patients had RT-PCR positive from conjunctival and nasopharyngeal swabs Figure 3.46–50
This literature review analizes the presence of the virus in asymptomatic and symptomatic patients. The mechanisms of transmission of SARS-CoV-2, the viral kinetics and antibody response could predict outcomes; determine guidelines to reduce spread of infection and to adopt strategies for early detection in endoscopy. Guidelines of ASGE and ESGE for infection control during endoscopy alongside precautions for safety of personnel and patients state measures should be taken to avoid transmission of SARS-CoV-2, knowing the mechanisms could help to reduce the contact of instruments, environment and personnel in endoscopy room.49
None.
Author declares that there are no conflicts of interest
None.

©2020 Cortés, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.