eISSN: 2373-6372


Clinical Paper Volume 11 Issue 6
1Department of Internal Medicine, Islamic Azad University, Iran
2Student Research Committee, Shiraz University of Medical sciences, Iran
3Medical School, Isfahan University of Medical Sciences, Iran
4School of Medicine, St. George University, England
5Department of Orthopaedic Surgery, Massachusetts General Hospital, USA
Correspondence: Alireza Ebrahimi, Student Research Committee, Medical School, Shiraz University of Medical sciences, Karimkhan Zand St., Shiraz, Iran
Received: November 03, 2020 | Published: December 16, 2020
Citation: Rafiei R, Ebrahimi A, Bahrami M, et al. Sequential therapy for Helicobacter pylori infection with and without probiotics. Gastroenterol Hepatol Open Access. 2020;11(6):231-234. DOI: 10.15406/ghoa.2020.11.00444
Background: Helicobacter pylori (H. pylori) infection is considered to be among the most common infections all around the world. Previous studies have shown that H. pylori is gaining resistance to several antibiotic agents. This prompted the clinicians to prescribe various combined therapies, including, probiotics to overcome the drug resistance. The aim of this study was to determine whether probiotics can increase the eradication rate of H. pylori infection.
Materials and methods: This control trial was carried out on 106 H. pylori positive patients, assigned into two groups. Group A was treated with Amoxicillin, Pantoprazole, Tinidazol and Clarithromycin; group B was treated Familact capsules consisted of probiotics added to the same regimen administered to group A. At the end of treatment, urease breath test was done for the participants, and reported as positive or negative. T-test and Chi-square test were used for evaluation of the results, and P-value <0.05 was considered as significant.
Results: H. pylori eradication rate in group B (88.5%) was significantly higher than group A (63.3%). Besides, the participants who received probiotics seemed to show lower side effects, including, bad taste and epigastric pain.
Conclusion: The addition of probiotics to the sequential therapy could significantly increase H. pylori eradication rate.
Keywords: helicobacter pylori, sequential therapy, probiotics
Helicobacter pylori (H. pylori) infection is considered to be the most frequent infection involving gastrointestinal (GI) system, worldwide. The prevalence of H. pylori infection is measured to be 70–90% in the developing countries, and 25–50% in the developed areas.1 The disease can also lead to several upper GI diseases, including, chronic gastritis, and gastric malignancies.2 These facts, besides, the problem of raising antibiotic resistance had prompted the clinicians to vigorously try to find a more effective therapy for the infection. Sequential therapy is among the most common recommended treatment for H. pylori infection, and is consisted of a 10-days course of proton pump inhibitor (PPI), plus, 3 proper antibiotics, given in sequence.3 Previous studies showed that this regimen could lead to higher rates of H. pylori eradication in comparison with the conventional triple therapy.4–7 However, the antibiotic-associated GI side effects, including, nausea, diarrhea, bloating, vomiting, and abdominal pain, are considered among the major problems of the H. pylori therapies.8,9
Probiotics are microorganisms that decrease the harmful bacteria, and improve the intestinal microenvironment for digestion. These microorganisms could play a role in preventing and even eradicating H. pylori infection, according to the current evidence.10 As supplemental probiotics increased the eradication rates of H. pylori in previous investigations.11,12 While, there is still controversy in this regard, and some reports mentioned that the addition of probiotics does not have any significant impact on the eradication rates of H. pylori infection.13,14 Furthermore, the administration of probiotics was beneficial for decreasing the side effects of therapies, and increasing the treatment tolerance.12,15
Several studies have mentioned the positive outcomes of adding probiotics to standard triple therapy.8,12,15-23 While, the effect of adding probiotic to sequential therapy was not yet clearly investigated. In the present study, we aimed to evaluate the impacts of adding probiotics to the sequential therapy, and the side effects of this regimen.
This randomized-control trial was carried out on 106 H. pylori positive patients enrolled between June 2018 to June 2019 in Shariati hospital, Isfahan, Iran. The patients who underwent endoscopy because of different indications, and had positive H. pylori were considered eligible for the study.24 The inclusion criteria were as following: 1. Agreement on informed consent to participate in the study; 2. Age between 18 to 90 years old; 3. Not having used H. pylori eradication treatments prior to the investigation; and 4. Not having used the antibiotics that are prescribed in the investigation, over the last month.
The exclusion criteria were the patients’ unwillingness to enroll in the study or continue the treatment, not having the medical records of the patients, or comorbidities that affect the treatment process. The patients were enrolled in the study using available sampling method, and were randomly assigned into two groups. The group A was treated with Amoxicillin 1000 mg (two 500mg Capsule, Daana Pharma™, Iran) and Pantoprazole 40mg (Capsule, Abidi Pharma™, Iran) twice a day for 5days, then Pantoprazole 40mg, Tinidazol 500mg (Tablet, Abidi Pharma™, Iran), and Clarithromycin 500mg (Tablet, Daana Pharma™, Iran) twice a day for 5days aftermath. The same regimen was also used for the treatment of group B, with the addition of supplemental probiotic capsules (500mg, Familact, Capsule, Zist Takhmir™, Iran), consisting of Lactobacillus rhamnosus, Lactobacillus casei, Streptococcus thermophiles, Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacterium breve, and Bifidobacterium longum, twice a day for 10days. Urea breath test (UBT; Heliprobe® system, Sweden) was performed after 8 hours of fasting by a gastroenterologist for the participants, 1 week after 10days treatment period. The patients were visited during and after the treatment process, in order to evaluate any improvement of side effects of the regimen by a person who was blinded to the study.
The protocol of this investigation was approved by the medical ethics committee of the Medical School of Islamic Azad University of Najafabad, Iran (IRB number: Reg. No: ir.iau.najafabad.rec.1396.79). All results documented and analyzed by SPSS Software version 22.0. Data were depicted as means± SD and also frequency. Demographic data were analyzed by using chi-square and t test wherever appropriate. In order to compare data within and between the groups, student t test and paired t test were used, respectively. P-values less than 0.05 were considered as significant. The analysis of covariance test was applied for eliminating the impact of confounding parameters, both in the beginning and during the investigation.
52 out of 53 patients in group B (98.1%), and the entire patients in group A (100%) had completed the study. The exclusion of one participant from group B did not affect the study process, and the results of the tests. Demographic characteristics of the participants were summarized in Table 1. The group B was consisted of 25 (47.2%) male, and 28 (52.8%) female patients; and group A had 21 (31.6%) male, and 32 (60.4%) female patients. All participants were aged between 20 and 89 years old. There was no association between the eradication rate of H. pylori and the participants’ gender (p= 0.43) or age (p= 0.14). H. pylori eradication rate in group B (88.5%; n=46) was significantly higher than group A (63.3%; n=34) in group A (p=0.003). Figure 1 compares the eradication rate of H. pylori between the two groups.
|
Variables |
Groups |
|
||
|
A |
B |
P value* |
||
|
Gender |
Male |
39.6% (21) |
47.2% (25) |
0.43 |
|
Female |
60.4%(32) |
52.8%(28) |
||
|
Age |
|
42±12.7 |
46±16 |
0.14 |
Table 1 Demographic data
*P Value <0.05 was considered as statistically significant
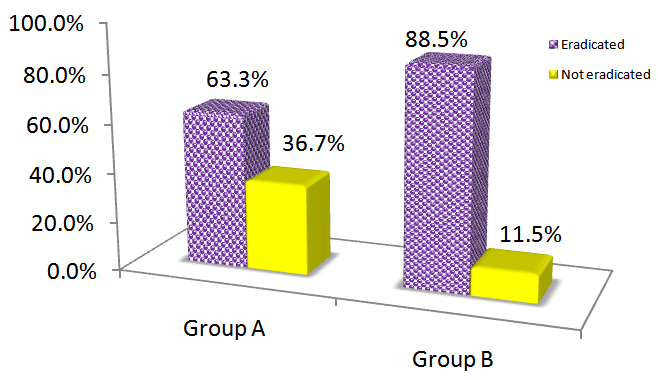
Figure 1 H. pylori eradication rate in Group A and Group B. Group B shows significantly higher H. pylori eradication rates, compared to group A (P<0.05).
Considering the side effects of sequential therapy, there were no significant differences in occurrence of headache, dizziness, itching, anorexia, bloating, fatigue, rash, nausea, vomiting, and constipation between the two groups (P=0.09). However, bad taste, and epigastric pain were considerably lower in group B compared to group A (p=0.005, and p=0.007). All the above-mentioned side effects were found to be mild, and well tolerated (Table 2).
|
|
Side effect |
Headache |
Dizziness |
Bad taste |
Itching |
Epigastric |
Anorexia |
Bloating |
Fatigue |
Rash |
Nausea & |
Constipation |
|
Group A |
31 |
2 |
1 |
17 |
1 |
0 |
1 |
2 |
2 |
1 |
4 |
0 |
|
Group B |
12 |
0 |
0 |
5 |
0 |
3 |
0 |
0 |
0 |
0 |
2 |
2 |
|
Pvalue* |
0.00 |
0.15 |
0.32 |
0.005 |
0.32 |
0.007 |
0.32 |
0.15 |
0.15 |
0.32 |
0.41 |
0.14 |
Table 2 Side effects of sequential therapy in participants
In our study, adding probiotics to the sequential therapy significantly enhanced the eradication rates of H. pylori. The result of present study is consistent with prior findings in this regard, as the 8-weeks administration of Lactobacillus gasseri was found to reduce H. pylori infection, and inflammation of gastric mucosa.25 Lactobacillus johnsonii was also had modulating impact on the H. pylori colonization in the pediatrics.26 Moreover, it has been mentioned that sixteen-weeks prescription of Lactobacillus johnsonii had decreased the H. pylori density, and the severity of antral gastritis.27 The addition of Bifidobacterium longum to the standard triple therapy was also resulted in higher eradication rates of H. pylori infection.28 Saccharomyces boulardii supplementation had also increased the H. pylori eradication rate.29 In this regard, a recent meta-analysis, consisted of 1469 subjects, reported that probiotic supplementation for patients who receive H. pylori therapies could significantly improve the eradication rate.30
The present study showed that adding probiotics to the sequential therapy could reduce the side effects of the treatment, as well. This also is consistent with prior evidence, since the addition of Lactobacillus rhamnosus GG to the standard triple therapy had reduced the occurrence of GI side effects.15 Besides, Bifidobacterium longum supplementation had reduced the frequency of diarrhea in the patients.28 Previous comprehensive investigations also mentioned that probiotic supplementation could decrease the occurrence of side effects, and increase the treatment tolerance by the patients.12,29–31
In the present investigation we prescribed a supplemental capsule of probiotics, consisting of Lactobacillus rhamnosus, Lactobacillus casei, Streptococcus thermophiles, Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacterium breve, and Bifidobacterium longum for the patients, which showed promising results. It has been mentioned that probiotic supplementation might reinforce gut innate defenses; as in vitro studies showed that Lactobacillus acidophilus could decrease the adherence of H. pylori to the enterocytes.32 Considering the different mechanisms of various strains of probiotics, researchers suggested that combination of distinctive strains could be useful for increasing the eradication rate of infection.33,34 However, more comprehensive studies mentioned that although multi-strain adjuvant probiotics can be beneficial as an additional therapy, combined probiotics could not significantly increase the efficacy.29,31
Not measuring the recurrence rate of infection in the participants to evaluate the long-term effects of the treatments is one of the limitations of this study. Moreover, not classifying the patients to age groups, and relatively low number of participants are the other limitations that could be mentioned. Another limitation is that the results may not apply to other countries and populations and further investigations on the effectiveness of these regimens should be performed on similar populations in different regions.
This study showed that the addition of combined probiotics to the sequential therapy could significantly increase the eradication rate of H. pylori, and reduce the side effects of treatment, particularly, bad taste and epigastric pain. However, further clinical investigations are still necessary to find the definite efficacy of the approach.
Ethics approval and consent to participate
Informed consent was obtained from all participants, their anonymity was guaranteed, and the study protocol was approved by the institutional research board (IRB) of Islamic Azad University of Najafabad, Isfahan, Iran (Reg. No: ir.iau.najafabad.rec.1396.79).
All authors of this article have been involved with the investigation and have approved the paper and agree to its submission and publishing in this journal.
The datasets used during the current study are available from the corresponding author on reasonable request.
The authors have no competing interest to declare.
The authors did not receive any financial help for this investigation.
Authors want to thank Medipress Co. and SIMR Co. for helping us in preparing and editing the draft of the paper and helping in finalizing the article.

©2020 Rafiei, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.