eISSN: 2373-6372


Clinical Paper Volume 15 Issue 1
1Richard Semelka Consulting, PLLC, USA
2Department of Imaging, Clinical Oncology and Hematology, Ribeirao Preto School of Medicine, University of Sao Paulo, Brazil
3Department of Radiology, Hospital Garcia de Orta, Portugal
4Department of Radiology, Hospital da Luz, Portugal
5Centre of Statistics and Applications of Faculty of Sciences of the University of Lisbon, Portugal
Correspondence: Richard Semelka MD, Richard Semelka Consulting, PLLC, 3901 Jones Ferry Road, Chapel Hill, NC 27516, USA
Received: February 04, 2024 | Published: February 16, 2024
Citation: Semelka RC, Elias JJ, Pereira JC, et al. Hepatic steatosis: additional findings in the splanchnic system on magnetic resonance imaging. Gastroenterol Hepatol Open Access. 2024;15(1):3-9. DOI: 10.15406/ghoa.2024.15.00570
Purpose: To document additional findings in the Splanchnic System in patients with hepatosteatosis on clinical MR studies.
Materials and Methods: One hundred randomly selected studies of patients with hepatosteatosis who underwent clinical MR studies during a six-month interval were included. Clinical information on the current requisition and prior abdominal studies within one year was recorded. No research or additional investigation into medical records was performed. Statistical analyses were performed.
Results: Among 100 patients with hepatosteatosis, there were 59 Females (mean 52.6 years). The presenting complaint was abdominal pain, right upper quadrant, or general pain in 77. Ninety-nine patients showed increased enhancement of the upper GI tract in at least one segment (esophagus and duodenum most commonly). There was a significant association between the subjective grading of hepatosteatosis and bowel enhancement in the Splanchnic System (p=0.00049). Ninety-four were overweight/obese; 23 had pancreatic steatosis; 8 had mesenteric panniculitis. Other findings were recorded.
Conclusions: Variable increased enhancement of the upper GI tract was present in almost all patients with hepatosteatosis, and various other abnormalities in the splanchnic system were observed. Hepatosteatosis and inflammation of the upper GI tract may be commonly present together and may be part of a larger picture of splanchnic abnormalities.
Keywords: hepatosteatosis, splanchnic system, mri, enhancement, pancreatic steatosis, mesenteric panniculitis
Hepatosteatosis is recognized as a widespread finding in the US population.1,2 Many investigators consider that hepatosteatosis may have already become the most common cause of chronic liver disease, with the attendant risk of hepatocellular carcinoma.3,4 Obesity has been widely studied as the precursor to hepatosteatosis.5 Metabolic Syndrome (MS) arises from visceral fat accumulation with lifestyle-related diseases linked with abnormalities in glucose metabolism, dyslipidemia, and hypertension. It has been recognized to be associated with hepatosteatosis, pancreatic steatosis, and increased intraperitoneal fat.6–9 The association between hepatosteatosis and pancreatic steatosis has been described.10 Little work has been performed to examine other associations of findings in the splanchnic system. Recent studies have also shown a high association with inflammation in upper gastrointestinal disease11 including the distal esophagus12,13 and stomach.14,15 As MS is a diagnosis based on metabolic clinical findings, it is uncommon that the term is used as an explanation for disease in imaging studies. MRI has been recognized for decades to represent the optimal diagnostic imaging tool to identify the presence of hepatosteatosis.16,17 MRI has also been shown to be optimal for evaluating the gallbladder and biliary tree,18 the pancreas19 and the spleen.20 MRI has established a primary role in investigating inflammatory disease of the small bowel and colon because of its superior ability to show increased enhancement in the setting of inflammation compared to CT.21–23
Despite the broad role that MRI has established for the investigation of hepatic and pancreatic steatosis, focal hepatic and pancreatic lesions, the biliary system, and the spleen on an individual disease basis, to our knowledge, no prior report has defined the extent to which hepatosteatosis is associated with other MR findings in the Splanchnic System. To our knowledge, no prior report has defined the extent to which hepatosteatosis is associated with other MR findings in the Splanchnic System. This report represents a preliminary description of observational findings in the splanchnic system in individuals with hepatosteatosis, interpreted on prospective reads of clinical cases in a private practice setting. We believe the umbrella term for these imaging findings should be an imaging description, Splanchnic Inflammatory Syndrome (SIS), rather than a metabolic condition, MS.
Population
This preliminary investigation was performed as a prospective clinical interpretative investigation by a single experienced radiologist (> 30 years of clinical and clinical research experience with modern body MR technique). Associated splanchnic system findings were described in individuals with hepatosteatosis (preliminary study). Diagnostic reports on consecutive patients were collected from January 2022 - May 2023, and 100 randomly selected reports were tabulated into a data set. Fifteen randomly selected anonymized cases were shown in a blinded fashion to a second radiologist with 16 years of clinical and clinical research experience in body MRI to ascertain the level of agreement of findings between observers. The second reviewer was instructed in advance about the grading of severity. All subjects were studied at outpatient imaging facilities in the greater New York City area. Additional findings in the Splanchnic System and the severity of the findings were recorded. Patient demographics, prior imaging studies, and clinical indications for MRI and prior studies were tabulated. No research was performed. Additionally, there was no interrogation of clinical records for medication use or other relevant information and no directed communications to referring practitioners. Information from contact initiated by referrers was recorded, but this was not systematically performed in all patients and hence did not appear in the Results.
Image acquisition and interpretation
No novel sequences are necessary to evaluate SIS. All studies were performed as multisequence/ multiparametric studies. The sequences include a 3D T1-weighted gradient echo sequence (Dixon, selective-excitation, or spoiled technique), fat- and non-fat-suppressed T2-weighted spin-echo variants, and diffusion-weighted imaging. Extracellular Gadolinium-based contrast agent (GBCA; Clariscan, GE Medical Systems, Rochester, Minnesota) at the standard dose (0.1 mmol/kg) was administered (arterial, portal-venous, and interstitial phases). The one non-conventional approach was using the 5-minute postcontrast T1-weighted sequence to evaluate venous phase enhancement. One board-certified radiologist with more than 30 years of clinical and clinical research experience with contrast-enhanced body MRI interpreted all studies in a private clinical practice setting.
Subjective fat grading in the liver was done as follows:
Determination of enhancement of the upper GI tract was considered indicative of active inflammation. This employed the approach similar to the approach validated for patients with Crohn's Disease24 and was done as follows:
The diagnostic criterion for hepatomegaly was based on unidimensional measurement (midclavicular line greater than 15.5 cm) and other features. Pancreatic steatosis was detected based on chemical shift imaging and fat-suppressed images. Mesenteric panniculitis was determined using fat-suppressed T2-weighted images and out-of-phase T1-weighted images. Gallbladder disease (including cholelithiasis and mural enhancement), focal liver lesions, and pancreatic cyst detection followed standard subjective approaches. The subjective assessment of obesity was made on visual fat assessment based on two major findings to describe obesity: anterior subcutaneous fat > 2 cm in thickness at a peri-umbilical level and flank fat at a level of superior iliac crest > 5 cm. All the procedures were conducted strictly following the Declaration of Helsinki. Since this is a retrospective observational report, in which no research was performed and collected data was stripped of all personal identifiers, informed consent was not obtained.
Statistical analysis
Descriptive statistics were performed to describe and summarize the basic features of the study’s data. To evaluate if there was an association between hepatosteatosis and bowel enhancement in the Splanchnic system, Fisher’s Exact Test was used as the assumptions for the Chi-Square test were not met (more than 20% of the expected cells had values less than 5). As the resulting table was not 2 x 2, Fisher’s Exact Test using Monte Carlo simulations (with 2000 replicates) was used. All analyses were performed using the R software for statistical analysis (version 4.2.1 – 2022). R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
In the preliminary study, among 100 patients with hepatosteatosis, there were 59 Females (all patient-mean aged 52.6 years and an age range of 19-65). The presenting complaint was abdominal pain, right upper quadrant, or general pain in 77 (65 presented with generalized abdominal pain, 10 with right upper quadrant (RUQ) pain, and 2 with left upper (LUQ) pain). Ninety-nine patients showed increased enhancement of the upper GI tract in at least one segment (esophagus and duodenum most commonly). The central observation was that increased upper GI enhancement of some combination of distal esophagus, distal stomach, proximal duodenum, and jejunum was essentially always present in the setting of hepatosteatosis, observed in 99/100 reported cases (Figure 1) (Table 1).
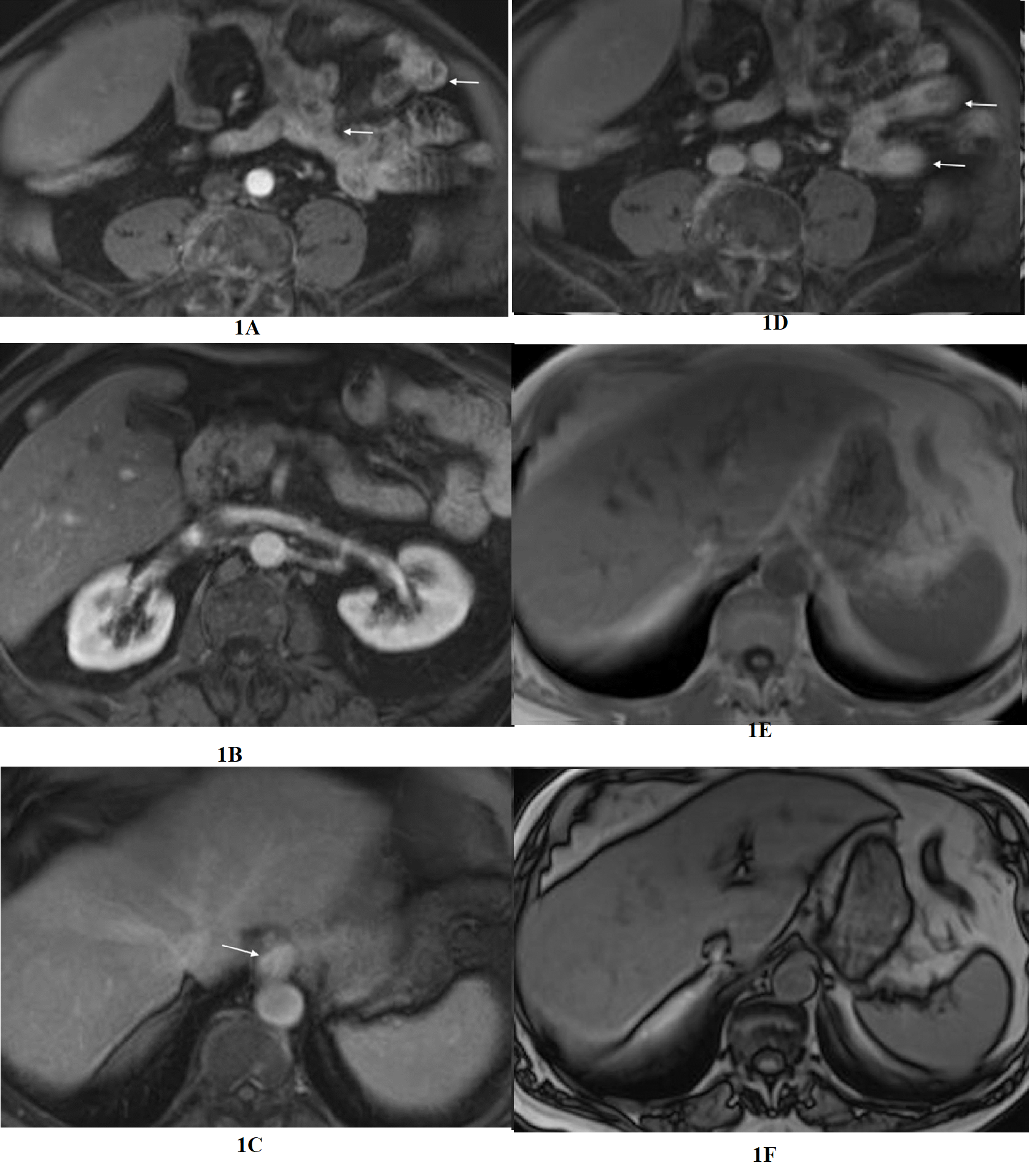
Figure 1 Evaluation of the upper abdomen of a patient with clinical diagnosis of irritable bowel syndrome. Axial T1-weighted images acquired in the arterial phase (a,b) and the interstitial phase (c,d). Moderate severity increased enhancement of the distal esophagus is appreciated in the interstitial phase (arrow, c). Duodenal inflammation is also depicted with increased enhancement in the interstitial phase (arrow, d). The enhancement approximates the intensity of adjacent IVC and aorta. Increased esophageal enhancement is most easily appreciated of all the upper GI segments, likely due to its fixed and consistent location. In-phase (e) and out-of-phase (f) images of the liver, exhibit normal signal relationship of liver and spleen on in-phase, but on out-of-phase signal of liver and spleen are approximately the same, which reflect mild hepatosteatosis.
|
Increased enhancement |
Number of patients |
|
Esophageal |
76 |
|
Stomach |
63 |
|
Duodenal |
90 |
|
Jejunal |
45 |
Table 1 GI segment exhibiting increased mural enhancement
There was a significant association between the subjective grading of hepatosteatosis and bowel enhancement in the Splanchnic System (p=0.00049). Among the 100 patients, 81 had liver fat and bowel enhancement measurements within 1 grade, as follows: Thirty-three with minimal hepatosteatosis, 18 with minimally increased bowel enhancement, 15 with mild, 32 with mild hepatosteatosis, 26 with mildly increased bowel enhancement, 2 with minimal, and 4 with moderate; 15 with moderate hepatosteatosis: 6 had moderately increased bowel enhancement, 9 had mild; one with moderately severe hepatosteatosis and moderate hepatosteatosis. No subject with severe hepatosteatosis had concordant increased bowel enhancement within 1 grade. Among the 19 discordant cases, 2 had minimal hepatosteatosis with moderately increased bowel enhancement; 1 with mild hepatosteatosis had normal bowel enhancement; 7 had moderate hepatosteatosis with minimally increased bowel enhancement; 8 with moderately severe hepatosteatosis, 1 had minimally increased bowel enhancement, and 7 had mild; 2 with severe hepatosteatosis had moderately increased bowel enhancement.
RUQ pain, as a described complaint on the image requisition, commonly was not associated with gallbladder disease; instead, duodenal inflammation was present in all cases (Figure 2). Five patients complaining of RUQ pain had a normal gallbladder and biliary tree (Table 2). Pancreatic steatosis was present in 23 patients (3 with cysts). Mesenteric panniculitis was present in 8 patients. It was always associated with increased jejunal inflammation (Figure 3). 11 subjects had Irritable bowel Syndrome in the clinical description, and 8 also mentioned gastro-esophageal reflux. 94 were overweight/obese; 77 had hepatomegaly; 23 had chronic liver disease/ cirrhosis, 16 had liver cysts/ biliary hamartomas; 16 had hemangiomas; 15 had pancreatic cysts; 14 had splenomegaly, 10 had focal nodular hyperplasia (FNH); and 7 had cholelithiasis. A full description of the additional findings is presented in Table 3. The clinical information provided on the MR requisition, or requisitions of imaging studies preceding the MRI, was 65 patients with generalized abdominal pain, 10 with right upper quadrant (RUQ) pain, of which 5 had normal gallbladder and biliary tree (Table 3); 2 with LUQ pain (both with increased jejunal enhancement); and 23 the original reason for the patient being imaged for abdominal disease not described, and only follow-on requests such as evaluate liver lesion. The blinded review of 15 anonymized cases by a second reader showed 3 cases of disagreement of 1 grade between the interpretations of bowel findings.

Figure 2 Fatty liver with multiple focal nodular hyperplasias with simultaneous upper GI inflammation. Axial T1-weighted images acquired in the arterial phase (a) and the interstitial phase (b). Increased mural enhancement of the stomach, duodenum, and jejunum is observed in the interstitial phase (arrows, b). Mild increased enhancement of the jejunum is shown as segments of jejunum where mural thickness is 4 mm and mural enhancement exceeds the enhancement of paraspinal muscles and approximates the enhancement of vessels and renal parenchyma. Moderately increased enhancement of the gallbladder is shown as enhancement of the gallbladder wall that shows progressively increased intensity from arterial phase to interstitial phase enhanced images (open arrow, b). In some cases, the increased gallbladder wall enhancement was concomitant with inflamed duodenum shown to abut the gallbladder. Incidental gallbladder polyps were observed. Coronal venous phase image (c) shows the increased duodenum and jejunal mural enhancement. Several uniform enhancing lesions in liver represent FNHs.
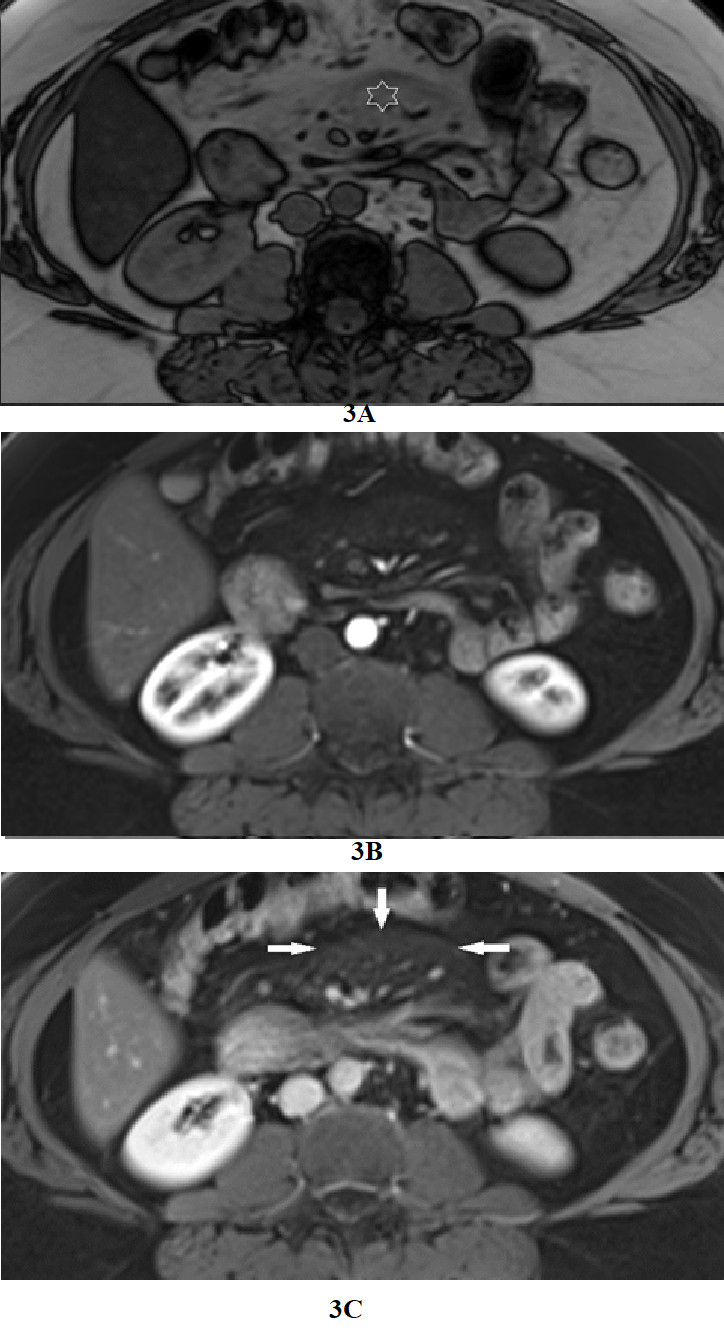
Figure 3 Increased jejunal enhancement in mesenteric panniculitis. Axial T1-weighted out-of-phase (a), arterial phase (b), and interstitial phase (c) images. Out-of-phase images (a) exhibited the greatest clarity for depicting mesenteric panniculitis. On out-of-phase images, the mesenteric fat is mild gray in signal (star) and has definable margins against a background of higher signal peritoneal fat. Occasional subcentimeter lymph nodes are commonly seen. Note on arterial phase image the mesenteric panniculitis is only minimally evident, and the enhancement of bowel is relatively unremarkable compared to interstitial phase images. This process is also shown as a mildly increased enhancement of mesenteric fat with definable margins on interstitial phase (arrows, c) images. Increased duodenal and jejunal enhancement is evident in interstitial phase images (c).
|
Number of patients |
MR findings |
|
5 |
Normal GB and Biliary ducts |
|
2 |
Cholelithiasis |
|
1 |
Subacute acalculous Cholecystitis |
|
1 |
Subacute Calculous cholecystitis |
|
1 |
Cholecystectomy with normal biliary ducts |
Table 2 Gallbladder and biliary findings in individuals with RUQ pain
|
Number of patients |
Additional findings |
|
94 |
Overweight/obese |
|
77 |
Hepatomegaly. |
|
23 |
Chronic liver disease/ cirrhosis |
|
23 |
Pancreatic steatosis |
|
16 |
Liver cysts/ biliary hamartomas |
|
16 |
Hemangiomas |
|
15 |
Pancreatic cysts |
|
14 |
Splenomegaly |
|
10 |
Focal nodular hyperplasia |
|
8 |
Mesenteric panniculitis |
|
7 |
Cholelithiasis |
|
4 |
Regenerative and dysplastic nodules |
|
4 |
Increased mesenteric fat |
|
4 |
Subacute cholecystitis |
|
3 |
CBD dilation, no choledocholithiasus |
|
2 |
Gallbladder adenomyomatosis |
|
1 |
Chronic pancreatitis |
|
1 |
Liver metastases |
|
1 |
Hepatocellular carcinoma |
|
1 |
Pancreatic cancer |
Table 3 Additional findings in the splanchnic system in individuals with hepatosteatosis
The Splanchnic System is a relatively self-contained system of organs involved in the digestion and processing of food to provide nutrition to the entire body. The gastrointestinal tract is the intake organ and the one structure in direct contact with the external environment, the upstream source. The pancreas, gallbladder, and biliary tract are involved with the processing. The spleen stores immune cells for the splanchnic and entire body systems. The liver is the final downstream organ in the splanchnic system and acts as the clearinghouse of all nutrient elements and detoxification. Based on the association between hepatosteatosis and increased enhancement of the upper GI tract, our opinion is that the GI tract, as the entry point to the system, is possibly the root cause of many inflammatory processes throughout the Splanchnic System. This includes many benign disease processes of organs in the system; for example, disorders of the pancreas, gallbladder, and biliary tree may have as their root cause in GI tract inflammation. Organs have relatively limited responses or reactions to chronic inflammatory conditions. Steatosis is a typical response to inflammation, such as hepatosteatosis, pancreatic steatosis, excess mesenteric fat, and mesenteric panniculitis.10,25,26
This constellation of imaging findings comports with MS. Critical elements, including hepatosteatosis, pancreatic steatosis, and increased peritoneal fat, have been shown to represent imaging biomarkers.6–9 Obesity, present in 94% of patients in our preliminary report, is a state of low-grade chronic inflammation that causes multiple metabolic diseases. During obesity, signaling via cytokines of the TNF family mediates cell death and inflammation within the adipose tissue, eventually resulting in lipid spillover, glucotoxicity, and insulin resistance.27 Obesity is an independent risk factor for developing hiatal hernia and gastro-esophageal reflux disease28 which likely accounts for many patients showing distal esophagus enhancement in our report. Recent studies have shown a relationship between erosive esophagitis and MS. In a case–control study including 1679 cases of erosive esophagitis, multiple regression analysis of various factors showed that MS was a significant independent risk factor.12 Suggested that intraesophageal damage may be a dynamic and migratory process in which MS is associated with accelerated progression to or attenuated regression from erosive states.13 76 (76%) of subjects in our report showed increased esophageal enhancement. Several studies have also reported a relationship between obesity and histologic gastritis, defined as the presence of inflammation of the gastric mucosa.14 63 (63%) of subjects in our report showed increased gastric wall enhancement.
Regarding duodenal and jejunal enhancement, nonalcoholic fatty liver disease (NAFLD) is known to be associated with gastroduodenitis and other inflammatory bowel diseases.29 90 (90%) and 45 (45%) of subjects in our report showed increased duodenal and jejunal enhancement, respectively. One exciting prospect is that the abnormally increased mural enhancement we have described may reflect cellular processes such as cytokine release and hormone production. The prevalence and development mechanisms of pancreatic steatosis in patients with metabolic disorders remain unclear. However, a recent systematic review and meta-analysis showed that nonalcoholic fatty pancreas disease is significantly associated with an increased risk of MS and its components.30 In our series, 23 (23%) individuals showed pancreatic steatosis. A recent retrospective study by Gunes et al.31 reported an increased occurrence of MS in 102 patients with mesenteric panniculitis compared to 408 matched controls. Interestingly, all 8 (8%) cases of mesenteric panniculitis in our preliminary report were accompanied by increased jejunal enhancement (Figure 3). A parallel finding is found in Crohn's disease with the hypertrophy of the mesenteric fat (fibrofatty proliferation) adjacent to the mesenteric border of the diseased bowel segments.32
Another interesting association, present in our preliminary group and consistently observed in our extended clinical practice, is that irritable bowel syndrome (IBS) was a common clinical indication in subjects that showed findings of SIS on MRI. All eleven patients with a clinical history of IBS showed increased enhancement of the jejunum, and eight also had distal esophageal enhancement. The relationship between MS, IBS, and SIS requires further investigation. It may be that the clinical diagnosis of IBS often reflects the GI component of the clinical entity MS. Based on our targeted preliminary group, expanded real-world clinical diagnostic observations, and current literature on MS, our theory is that SIS represents the Splanchnic System findings of MS. We also opine that these features may reflect not only clinically mature MS but also early stages and probably pre-clinical MS. The imaging presence of upper GI inflammation and hepatosteatosis may precede clinical MS. The explanation why the association between these imaging findings of various organs and tissues in the splanchnic system has not been previously established in over 30 years of modern body MRI may be ascribed to several factors: 1) current short duration high spatial resolution MR sequences with contrast enhancement is uniquely able to see inflammation of all the organs and tissues involved. Meanwhile, US and CT, which are more commonly used to evaluate outpatients with abdominal disease, have limitations. Ultrasound is unreliable in visualizing the bowel and inconsistently demonstrates the pancreas. While CT is effective at showing anatomical findings, it is relatively poor at showing increased mural enhancement of the bowel; 2) in most academic centers, outpatients without cancer presenting with abdominal pain have been an uncommon indication for MRI. In this report, all subjects were imaged in an outpatient clinic setting. The outpatient population in this report may more closely reflect the general public and what the relative occurrence is of various abdominal diseases as a whole, compared to patients seen in the hospital setting; 3) clinical proof for the validation of the entire imaging picture likely would require biochemical proof.33
Future investigations should delve into processes uncovered in this clinical catalog of findings. For example, cystic change can result from inflammation that causes vacuolization of tissue with cyst formation; therefore, to what extent are pancreatic cysts and hepatic cysts/biliary hamartomas associated with upper GI inflammation? The association of other benign liver lesions with SIS should be considered. Acalculous cholecystitis may be particularly interesting. A prior report considered a systemic nature to acute acalculous cholecystitis34 which we postulate may be duodenal inflammation. Increased enhancement of the duodenum was not uncommonly associated with inflammation of the gallbladder wall, especially when they were in physical contact (Figure 2). It is conceivable that GB wall inflammation in acute or acute-on-chronic acalculous cholecystitis may represent a sympathetic response to adjacent duodenal inflammation. This may also apply to entities under the biliary dyskinesia rubric and Sphincter of Oddi Dysfunction.35,36 In fact, of the ten patients who presented with RUQ pain, 5 had normal gallbladder and biliary tree, and 1 had cholecystectomy with a normal biliary tree.
There are important limitations to this report. Firstly, it is an observational report with randomly selected cases that involve no research. The report, however, provides our opinion based on controlled reporting and broader real-world clinical experience. Although very interesting, these findings should be considered preliminary and require controlled future research to verify or refute them. A further cautionary note is that some individuals are at risk of showing prolonged toxicity to GBCA exposure, in particular, Gadolinium Deposition Disease. Hence, we do not recommend that everyone with obesity and abdominal pain should undergo an MRI with a GBCA injection. DWI might be valuable in cases where GBCA is not administered. Interestingly, a collective of global hepatologist experts have recently introduced new nomenclature for hepatosteatosis in persons who are overweight or obese or have type 2 diabetes or metabolic dysregulation, regardless of the coexistence of excessive alcohol consumption and other chronic liver disease37 so our redefining of splanchnic system imaging findings is quite topical.
An essential piece of information that needs to be included is whether some patients are on various medications, such as gastric acid-suppressing/mediating drugs. Their use may explain some discordance between greater hepatosteatosis and upper GI inflammation. There also was no systematic comparison of MR findings with other techniques, such as endoscopy. It is unclear if minimal findings of increased upper GI enhancement are identifiable on endoscopy. As a result, in clinical interpretations, we avoid emphasizing minimal upper GI tract increased enhancement unless there is a history of abdominal pain. In 23 patients, the original reason for abdominal imaging was unknown because no investigation on the part of the authors was performed beyond looking at clinical information provided in the present MR study and immediately preceding other imaging studies. Abdominal pain was the indication for imaging in this report in 77% of the patients, which concurs with a prior study that described abdominal pain as the most common indication for abdominal imaging in the ambulatory patient population.38 This report introduces the concept of a constellation of inflammatory changes involving the splanchnic system, which we term SIS. Our opinion is that this represents the splanchnic system findings of MS. The entire constellation of findings, especially of upper GI segments of SIS, to the present, has yet to be reported in imaging studies. MS is highly under-reported, even in the clinical indication for imaging. Based on our preliminary study and larger real-world experience, we consider it imperative that when abdominal MR studies of the abdomen are reported, attention should be paid to and reported on the following: hepatic and pancreatic steatosis and upper GI enhancement findings should be routinely described, and attention must be paid to the possible presence of mesenteric panniculitis.
Imaging findings in the Splanchnic System observed in the setting of hepatosteatosis show an increased enhancement of segments of the upper GI tract that frequently coexist with inflammatory changes that affect other organs in the Splanchnic System. Our findings support that there may be a broader association between conditions afflicting organs and tissues in the Splanchnic System, and we propose the designation Splanchnic Inflammatory Syndrome.
None.
The author declares that there are no conflicts of interest.

©2024 Semelka, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 IBS awareness month is April, which is very common syndrome globally, so to develop awareness about the irritable bowel syndrome (IBS), we are eager to publish articles to spread acquaintance to all our readers. So, take the opportunity in sending your valuable articles to raise alertness to the global people and also get the best discount of 30% for your submissions to our Gastroenterology & Hepatology: Open access (GHOA).
IBS awareness month is April, which is very common syndrome globally, so to develop awareness about the irritable bowel syndrome (IBS), we are eager to publish articles to spread acquaintance to all our readers. So, take the opportunity in sending your valuable articles to raise alertness to the global people and also get the best discount of 30% for your submissions to our Gastroenterology & Hepatology: Open access (GHOA).