eISSN: 2373-6372


Research Article Volume 14 Issue 1
1Pharmacology Department, Medical Research and Clinical Studies Institute, National Research Centre, Egypt
2Institute of Molecular Biology and Biotechnology (IMBB), The University of Lahore (UOL), Pakistan
Correspondence: Manal ME Ahmed, Department of Pharmacology, Medical Research and Clinical Studies Institute, National Research Centre, Giza, Egypt, Tel +20-10936-27027
Received: December 20, 2022 | Published: February 9, 2023
Citation: Ahmed MME, Noureen A, Rafiq A. Generation of monoclonal antibodies against PBP2a to improve diagnosis and potential treatment of MRSA infections. Gastroenterol Hepatol Open Access. 2023;14(1):14-17. DOI: 10.15406/ghoa.2023.14.00536
Background: MRSA is a type of MDR bacterium. All around the world, it leads to significant nosocomial and community-acquired diseases. The discovery of fresh ideas is very important because there are so few effective treatments for MRSA infections. PBP2a is a great candidate for the development of MRSA-specific Mabs.
Aim: The purpose of this study was to create murine Mabs against PBP2a in order to enhance MRSA laboratory detection and possibly treatment.
Methods: Anti-PBP2a MAb was tested for biodistribution, therapeutic, and preventative effects in comparison to vancomycin. After receiving anti-PBP2a MAb therapy, biodistribution was assessed between 12 and 96 hours later.
Results: During the observation timepoints, the majority of the generated MAb stayed in the serum and just a tiny fraction was found in the kidneys, lungs, and spleen. A prophylactic experiment in mice given the lethal dosage 50 (LD50) found that treated animals with anti-PBP2a MAb had a greater survival rate than the control group. In a therapeutic experiment, one dosage of anti-PBP2a MAb reduced bacterial load in the kidneys similarly to the group treated with five doses of vancomycin, and a combination therapy with anti-PBP2a MAb and vancomycin was more effective than either drug used alone.
Conclusion: The findings of this study suggested that an anti-PBP2a MAb may be extremely useful in the creation of innovative diagnostic tools and promising treatments for preventative and therapeutic measures against MRSA infections.
Keywords: MRSA, MDR, Mabs, prophylaxis, treatment, biodistribution
Staphylococcus aureus (S. Aureus) is representing a considerable disease burden. Deep wound infections, bacteremia, infective endocarditis, pneumonia, osteomyelitis, and enterotoxin-mediated shock are among the life-threatening illnesses it can cause.1 Methicillin-resistant Staphylococcus aureus (MRSA) is a very significant and widely distributed member of this species. Since there is currently no effective S. aureus vaccine on the market, basic and clinical studies show a great need for developing protective vaccines against S. aureus in general and MRSA in particular.2–4 The following succinctly describes the difficulties in developing a S. aureus vaccine: The natural immunity against S. aureus is very weak, the pathogen- derived antigens are not immunogenic, and S. aureus has many virulence and immune evasion factors in addition to redundant nutrition acquisition pathways. The difficulties impede the creation of a protective S. aureus vaccine. The fundamental concept of protection offered by vaccine techniques typically rely on preventing pathogen interactions by neutralising antibodies with, entering host cells, or detoxifying virulence components embodied by the pathogen. Due to the nasal colonisation of S. aureus, the majority of adults have sufficient levels of circulating antibodies against a variety of staphylococcal antigens, which can offer some protection against the development of S. aureus.5,6 The most popular methods involve neutralising S. aureus toxins or surface antigens to induce opsonizing antibodies, but sadly, clinical trials have not shown that these methods are very effective. Despite playing important roles in host-pathogen interactions, such as host cell adhesion, extracellular matrix protein binding and degradation, iron uptake, or host fibrinolytic system intervention, S. aureus proteins failed to work as a target for vaccines generation.2–4 Preclinical and clinical studies’ findings showed that while immunisation with various S. aureus antigens always produces high antibody titers, it does not confer protection against S. aureus challenge7, 8. Although there are no overt safety issues with S. aureus vaccines, the information that is currently available on protective, non-protective, and disease-enhancing B- cell epitopes provides a framework for more precise vaccine development. Due to all of these factors, the development of monoclonal antibodies (MAbs) for preventative and therapeutic purposes has garnered a lot of interest9. Since MAbs can differentiate between protective and non-protective epitopes and can aid in the development of immunofocused antigens, they are now a crucial component of “reverse vaccinology”.10,11 The major goal of an epitope-focused vaccination is to increase the amount of immunogenic precision, which will increase the vaccine’s efficacy and safety profile. This method has been successful in creating RSV vaccine that is highly resistant to conventional vaccines in the recent past.12 Here, we sought to improve laboratory diagnostics and potential therapeutics by developing mouse MAbs against PBP2a.
Method: Mice
At Lahore University, female Balb/c mice were purchased and kept in customary laboratory settings. Mice were acclimated and given access to a standard diet ad libitum.
Growth conditions of S. Aureus strains:
At 37 °C, Luria Bertani broth (BD Difco, Germany), Mueller Hinton (MH)- or sheep- blood agar plates (OXOID, Germany) were used for culturing of Iberian Clone MRSA (purchased from the Unite´ des Agents Antibacte´riens—Institut Pasteur) and Staphylococcus aureus ATCC29213 (ATCC, USA).
MRSA immunogen:
Recombinant S. Aureus PBP2a expressed in E. Coli was acquired from RayBiotech as a 90% pure lyophilized powder for use as an MRSA immunogen.
BALB/c mice active immunisation with S. aureus immunogen
Incomplete Freund’s adjuvant (IFA) and complete Freund’s adjuvant (FA) was combined with recombinant S. aureus PBP2a expressed in E. Coli. A group of 10 mice (8–9weeks old) received two booster subcutaneous (s.c.) injections of 10ug immunogen formulated with IFA at days 28 and 56 after receiving an intraperitoneal (i.p.) immunisation with recombinant S. aureus PBP2a expressed in E. Coli formulated with FA. The formulation was passed via a T-connector (Discofix- 3, Braun Melsungen AG, Germany) between two syringes at least 100 times to gain a stable emulsion.
Generation of monoclonal antibodies.13
Ten BALB/c mice were given the S. aureus immunogen vaccine before being exposed to S. aureus ATCC 29213 as previously mentioned. Mice were given 5ug of vancomycin per g of body weight on day 10 following infection (Sigma-Aldrich, Germany). Prior to the development of hybridomas, mice were additionally given three consecutive days of s.c. administration of 10ug of immunogen without adjuvant. Spleens were removed, and, using Milstein and Köhler’s procedure with slight modifications,13 splenocytes were fused to Sp2/0-Ag14 myeloma cells. Briefly, 1.5x107 Sp2/0-Ag14 myeloma cells (DSMZ, Germany) and 5x107 spleen cells (Sigma-Aldrich, Germany) were combined, pelleted, and resuspended in 1.5ml of polyethylene glycol-solution before being placed in 20ml of Gibco RPMI medium (Thermo Fisher Scientific, Germany). In 5ml FBS, cells were pelleted and resuspended prior to supplementing with 45ml RPMI medium, 1 × Gibco L- GlutaMAX (Thermo Fisher Scientific, Germany), 10% FBS (Biowest, USA), 10% BMCondimed H1 (Sigma-Aldrich, Germany), 1 × Gibco HAT medium supplement (Thermo Scientific, Germany) and 24µM Gibco ß-Mercaptoethanol (Thermo Fisher Scientific, Germany). Before centrifuging and resuspending the cells in the HAT medium supplemented with 1.2% methyl cellulose (Sigma-Aldrich, Germany), the cell suspension was kept for 16hours in a CO2-incubator. Ten cell culture dishes were filled with cell suspension, which was then incubated for 12days in a CO2- incubator without touching. 96-well plates containing visible colonies were then used to conduct an ELISA test to check for the development of antigen-specific antibodies.14,15 Each monoclonal antibody’s IgG-subclass was determined13 in accordance with the manufacturer’s instructions using the isotyping kit IsoStrip (Roche, Germany).
Antibody purification
While murine NS0 cells producing MAbs were cultured in DMEM (Thermo Fisher Scientific, Germany) supplemented with 5% FBS (Biowest, USA) and 200nM methotrexate (Sigma Aldrich, Germany) at 37 °C in a humidified incubator and 5% CO2, we cultured hybridoma cell clones secreting various MAbs in hybridoma serum-free medium(H-SFM, Gibco, Thermo Fisher Scientific, Germany). We removed the cell culture supernatant by centrifuging it at 4000 X g for 10minutes at 4 °C while sterilising it with a 0.2m filter. An automated Profinia protein purification system (Bio-Rad, Germany) was used to purify antibodies using protein G (MAbs) (HiTrap protein G or A HP, Sigma Aldrich, Germany) affinity chromatography. antibodies were eluted using buffer and 400 mM glycine at pH 2.5 in accordance with the manufacturer’s instructions, and then exchanged to PBS using P-6 desalting cartridges (Bio-Rad, Germany). By measuring UV absorbance at 280nm, the antibody concentration was determined. The Roche (Germany) IsoStrip Mouse Monoclonal Antibody Isotyping Kit was used to determine the isotype of MAbs.
Evaluation of the Prophylactic effects of the developed MAbs against PBP2a:
Preparation of Iberian Clone MRSA
Iberian Clone MRSA was cultivated until it reached the exponential phase (OD600 0.6), after which it was washed and resuspended in sterile PBS at OD600 0.5, or around 4108 CFU (LD50). A 10-fold serial dilution was used to calculate this value. Bacteria were grown for 18hours at 37°C on Brain Heart Infusion (BHI) agar plates with 10ug/mL of oxacillin.
Survival assay
Dosage of 25mg/kg developed anti-PBP2a MAb for group (1) and PBS for group (2) were administered on the first day to female BALB/c mice that were 8 weeks old (n=10 per group, a total of 20 animals). Mice from both groups received an IP inoculation on day two that contained 4x108 CFU of the Iberian Clone MRSA. The mice were monitored for 10days in order to determine their survival rate.
Evaluation of the therapeutic effects of the developed anti-PBP2a Mab compared to vancomycin
Female BALB/c mice that were eight weeks old were separated into four groups, totaling 20 animals (n=5 per group). On the first day, an infectious dosage of Iberian Clone MRSA (7×106 CFU) was administered to each group. The mice were given doses of the developed anti-PBP2a MAb for group (1), vancomycin for group (2), Mab + vancomycin for group (3), and PBS for group (4) as a negative control, six hours after receiving the infective dose, as shown in the Table 1. The mice’s kidneys were aseptically removed for bacterial quantification on the fourth day. We kept an eye out every day during the experiment for any clinical symptoms or signs in the mice.
Group description
1 anti-PBP2a MAb (25mg/kg), administered IP on the first day
2 Vancomycin (7.5mg/kg), administered IM, 12/12 hours, 3 days (5 doses)
3 Combination (anti-PBP2a MAb + vancomycin) as previously described to groups 1 & 2
4 PBS (negative control), administered IP on the first day
Bacterial quantificationAfter being removed from the body, the kidneys were homogenised in 1mL of sterile Luria Broth (LB), serially diluted 10-fold, and then plated. The number of expanding colonies was counted in order to determine the bacterial concentration present in the kidneys.
Biodistribution of anti-PBP2a MAb in serum and organs
We employed female BALB/c mice that were 8 weeks old (n=20) to assess how the generated anti-PBP2a MAb was distributed in the serum and different tissues. Mice were IP-injected with either PBS (negative control) or anti-PBP2a MAb at a dose of 25mg/kg. At various intervals following injection (PBP2a-MAbs: 12, 24, 48, 72, and 96h; PBS: 96h; a minimum of three mice for each interval), serum samples, lungs, spleens, and kidneys were obtained. The tissues we extracted were homogenised. With various modifications for anti-PBP2a MAb quantification, an enzyme-linked immunosorbent test (ELISA) was carried out.14,15 Briefly, coating plates were performed using recombinant S. aureus PBP2a expressed in E. Coli at 1.0mg/mL, diluted in the coating buffer (0.1 M carbonate/bicarbonate coating buffer; pH 9.6), and incubated for an overnight period at 4 °C. The plates were blocked for one hour at 37 degrees Celsius after being rinsed four times with PBP 0.05% Tween-20 from Sigma-Aldrich. Serum or homogenised tissues were added to the plates and incubated there for 30minutes at 37°C.
The plates were then cleaned and treated with a diluted version (1:30.000) of the secondary antibody (anti-mouse polyvalent peroxidase-conjugated; Sigma-Aldrich) for 30minutes at 37 degrees Celsius. After washing the plates, chromogenic substrate solution was added for 10 minutes at room temperature to develop the reaction (TMB peroxidase, Bio-Rad, Hercules, CA). The reaction was stopped by adding 50mL/well of 2M H2SO4 and reading the optical density (OD) at 450nm on a microplate reader made by Benchmark (Bio-Rad). In order to make sure that neither endogenous antibodies nor other components of the samples will interfere with the analysis, a standard curve was built for each experiment using a previously measured MAb. A negative control mouse sample was added to the standard curve with the same volume and dilutions as the analyzed samples. Plotting the results on the standard curves allowed for the calculation of the amount of MAb present in each sample.
Statistical analysis
GraphPad Prism was used to do the statistical analysis (version 5). Using the Mann- Whitney U test, the bacterial burden was compared. Survival was represented as Kaplan-Meier curves, and log-rank (Mental-Cox) tests were used to analyze the data. An asterisk (*) is used in the figures to denote p-values that were < 0.05, which were considered significant differences.
Results of screening of supernatants from wells showing 30% or greater hybrid cell growth for PBP2a-MAb using ELISA:
On the 12th day after fusion, an ELISA assay was used to screen for anti-PBP2a MAb production utilising supernatants from hybridomas that covered 30% or more of the wells’ surface area. The findings showed that 15 of the 50 selected hybridomas were reactive to PBP2a. PBP2a had a strong reactivity with three hybridomas.
Characterization of anti- PBP2a Mab produced by the highly reactive hybridomas
Utilizing ELISA and immunoglobulin isotyping kits, the isotypes of three hybridomas’ secreted monoclonal antibodies were identified. One hybridoma was discovered to correspond to the IgG2b isotype and two hybridomas were discovered to correspond to the IgG2a isotype, respectively.
The result of survival after challenge with Iberian clone MRSA
To test the protective efficacy of the previously administered dose of anti-PBP2a MAb, Iberian Clone MRSA was used to challenge mice with LD50 (bacterial load sufficient to kill more than 50%). As shown in Figure (1), the rate of survival reached up to 70% (7/10 mice) compared to only 10% (1/10 mice) in the control group because prior treatment with anti-PBP2a MAb (prophylactic therapy) prolonged survival.
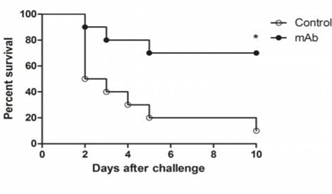
Figure 1 Effect of anti-PBP2a MAb as a prophylactic therapy in a challenge with a LD 50 of Iberian Clone MRSA (4x108 CFU), (p<0.05).
The result of therapeutic treatment anti-PBP2a MAb versus vancomycin
This experiment was designed employing the discovered anti-PBP2a MAb vs vancomycin in a therapeutic test because vancomycin is the initial antibiotic option for treating serious MRSA infections. MRSA strains were administered to BALB/c mice that treated 6 hours after infection. According to Figure 2, therapy with one dose of anti-PBP2a MAb resulted in a therapeutic efficacy level that was comparable to that for the group receiving five doses of vancomycin (92% versus 89% bacterial load reduction, respectively). Additionally, combination therapy that included both antibiotics and antibodies revealed a higher therapeutic level than single treatment, which could only reduce kidney bacterial load by up to 96% (Figure 2).
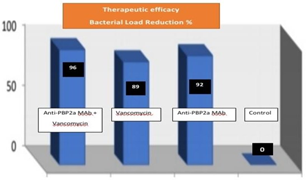
Figure 2 % of bacterial load reduction is in kidneys of mice treated with anti- PBP2a MAb, vancomycin, PBP2a Mab+vancomycin & control (p<0.05).
The result of biodistribution of anti-PBP2a MAb in serum and organs
The biodistribution of anti-PBP2a MAb and its penetration into tissues are crucial to study because MRSA infections can affect many body organs. ELISA was used to estimate the MAb levels in tissues at various periods. The outcomes demonstrated that anti-PBP2a MAb may access various tissues. Its concentrations in the serum, kidneys, lungs, and spleen were 73.2, 4.20, 2.12, and 0.67g/mL after 12 hours and 54.25, 2.80, 0.97, and 0.41g/mL after 96 hours, respectively. Tissue anti-PBP2a MAb concentrations were lower than serum levels. The mice used in this investigation had no clinical signs.
MDR bacteria are becoming more common at an alarming rate nowadays, and the treatment options are limited, making their infections a serious problem. One of the most significant MDR bacteria, MRSA has a high morbidity and few available treatments. Therefore, monoclonal antibodies used in passive immunotherapy are one of the most promising alternative techniques. MAbs are advantageous to prevent selective pressure, narrow-spectrum antibacterial therapies due to their lower toxicity and longer half-life. Although other studies using antibodies to target PBP2a confirmed their specificity for MRSA, they were ineffective.11 Our goal was to design an anti-PBP2a MAb. Our findings demonstrate that this MAb distinguishes native PBP2a protein on MARS isolates and binds tightly to recombinant PBP2a. We verified the therapeutic and protective efficacy imparted by the developed anti- PBP2a MAb in mice in comparison to vancomycin as the first antimicrobial choice based on bacterial load reduction in the kidneys and survival after challenge with a LD50. Using a prophylactic experiment in mice, the protection provided by anti- PBP2a MAb therapy was evaluated. The findings of the bacterial challenge test showed a considerable reduction in the amount of bacteria in the mice’s kidneys, as well as lower mortality and prolonged survival. Our findings are consistent with earlier investigations on DNA vaccination using the mecA gene11 and more current strategies involving the use of MAbs for preventative and therapeutic purposes.16
The structures, sizes, modes of action, and pharmacokinetics of monoclonal antibodies are distinct from those of antibiotics. The developed anti-PBP2a MAb’s biodistribution experiment in our investigation showed that the majority of MAb molecules remained in the serum, with only a tiny percentage being disseminated in other organs. Our findings concur with those of previous research.17 Patients who are being treated for MRSA infections at the beginning may find this feature helpful. MRSA has a high rate of morbidity, so treatment must start right away. The transpeptidase region of PBP2a is the area that the anti-PBP2a MAb mainly targets to impair the enzymatic role of PBP2a. . Combination therapy is also more successful than monotherapies. Here, combination antibiotic and antibody therapy led to a considerable decrease in the amount of bacteria in the kidneys.
We can advise the use of anti-PBP2a Mab in treating systemic infections caused by MRSA as well as for pre-operative patients that carry a significant risk for MRSA infections because MAbs of Ig G isotype characterize by an extended half-life.17
Finally, we have established the therapeutic and protective efficacy of anti-PBP2a MAb. Greater protection is offered by combining the developed MAb with vancomycin than by using either drug alone. The development of new diagnostic tools and novel therapeutics for preventative and therapeutic methods will greatly benefit from the use of anti-PBP2a MAb.
None.
Author declare there are no conflict of interest
None.

©2023 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.