eISSN: 2373-6372


Research Article Volume 11 Issue 6
1Department of Molecular microbiology, Federal State Budgetary Research Institution "Institute of Experimental Medicine" (FSBRI "IEM"), Russia
2Department of Nutrition physiology, Pavlov Institute of Physiology RAS, Russia
3Bioinformatic research, Saint Petersburg state University, Russia
Correspondence: Elena Ermolenko, Institute of experimental medicine (197376, Saint Petersburg, ul. Akademika Pavlova, 12), Russia, Tel 812 9533412625, Fax 8122349477
Received: October 14, 2020 | Published: November 9, 2020
Citation: Ermolenko E, Gromova L, Lavrenova N, et al. Effects of autoprobiotic consortium and fecal transplant on the digestive system and intestinal microbiota in the correction of experimental dysbiosis. Gastroenterol Hepatol Open Access. 2020;11(6):198-206. DOI: 10.15406/ghoa.2020.11.00440
Fecal transplantation and the introduction of indigenous bacteria (autoprobiotics) are currently in demand for the treatment of many diseases and accompanied by the development of intestinal dysbiosis and impairment of its functions. The specific effects of autologous (autoprobiotic) fecal microbiota transplants (AFMT) and a autoprobiotic consortium of indigenous fecal bacteria (AC) on the restoration of intestinal microbiota and activity of key digestive enzymes in Wistar rats with experimental dysbiosis was studied.
The composition of the fecal microbiome after correction of dysbiosis induced by ampicillin and metronidazole in Wistar rats of using AFMT (group F) and AC (group A) so as after introduction of phosphate buffer saline (group C1) was studied by metagenome analysis (16S rRNA) and qPCR comparing with normal microbiota (group C2, without consumption). The activity of alkaline phosphatase (AP), maltase (ML), and aminopeptidase N (APN) in the chyme of various intestinal parts was evaluated using biochemical methods.
The most significant changes of microbiota composition were observed in group C1: the representation of genera Lactobacillus, Ruminococcus and Prevotella spp. decreased, Proteobacteria, Families Enterobacteriaceae, genera Proteus spp., Klebsiella Enterobacter, Suterella, Serratia increased. The microbiome of animals from group F was characterized by a sharp increase in the representation of the phylum Bacteroidetes, genera Escherichia coli and Suterella sp., against the background of a decrease of phylum Firmicutes relative abundance. The representation of Lactobacillus spp., Enterococcus spp., Propionibacterium spp., Bifidobacterium spp. and Proteus spp. and E.coli), increased after the introduction of AC. Despite more pronounced residual changes in intestinal microbiome of rats from group C1, no significant changes in the activity of key digestive enzymes were detected. The exception was low AP activity in the duodenum chyme in group C1, possibly indicating insufficient protection from lipopolysaccharide of gram-negative bacteria. AP was higher in group A than in group C2 in the chyme of the colon. AMFT induced more sufficient changes in the carbohydrate and protein metabolism: the increase ML in small intestine and reduced of APN in the chyme in the virtually throughout the intestine.
Individual representatives of intestinal microbiota may affect the activity of digestive enzymes. This study has revealed the presence of relationships between the activity of ML in the chyme and the representation of Bacteroides spp. (direct correlation) and Prevotella spp. (inverse correlation). It is necessary to take into account the specific influence of AMFT and AC on microbiota and digestive function for choosing a means for the correction of intestinal dysbiosis.
Keywords: autoprobiotic, fecal transplantation, digestive enzymes, microbiome, chyme
AFMT, autologous fecal microbiota transplants; AP, alkaline phosphatase; ML, maltase; AC, anaerobic consortium
Multiple clinical studies and experimental models1–3 have demonstrated that probiotics are effective in correcting intestinal dysbiosis, which occurs in many medical conditions and results from various exogenous and endogenous causes. In some cases, however, probiotics themselves can induce dysbiosis, cause allergic reactions, dyspepsia or even infectious diseases.4–13 Because of the lack of biocompatibility between the host organism and its microbiota to the administered probiotics they are eliminated rapidly from the organism, and longer treatment periods are needed to achieve lasting results.1–14 The use of beneficial indigenous bacteria (autoprobiotics)15–19 or autologous fecal transplantation18 has high potential for overcoming these problems. The efficacy of mono-strain autoprobiotics has been proven in an intestinal dysbiosis experimental model14,18,20 in treating of irritable bowel syndrome, Parkinson's disease and when used with antibiotic treatments of pneumonia.1,4,16 However, the introduction of single components of intestinal microbiota cannot recreate the complete microbiota, characterized by a high biodiversity and the presence of its obligate representatives. Microbial fecal transplantation is preferable in this case, which has proven effect in treating pseudomembranous colitis, ulcerative colitis, multiple sclerosis, Parkinson’s disease and other diseases.6,21–23 Efforts are also under way to create artificial microbiota by administering various donor strains isolated from the intestines of healthy donors,23,24 as well as autologous strains or consortia grown from the patient’s own fecal samples collected before onset of the disease and its treatment.1 Switch from proteolytic to saccharolytic fermentation could be of major interest for the prevention and/or treatment of metabolic diseases. The gut microbiota is able to ferment indigestible carbohydrates (for example, dietary fibre), thereby yielding important metabolites such as short-chain fatty acids and succinate.25,26 Metabolic disorders and their connection with microbiota content to allow to use of probiotic or prebiotic therapy are discussed.27–30 However, microbiome-based treatments of digestive function destruction often report conflicting results, indicating a need for further research.
In our previous study, we used a model of experimental dysbiosis in rats to show the distinctive features of action of indigenous enterococci, lactobacilli, bifidobacteria and a consortium of fecal bacteria (AC) grown under anaerobic conditions1 on intestinal microbiota and immunity. However, a comparative study of microbiome composition and effect of autologous feces and AC on the digestive system has not been conducted.
The aim of this study was to compare the effect of autologous fecal transplant (AFMT) and the autoprobiotic consortium of bacteria (AC) on the recovery of microbiota and the activity of digestive enzymes in the intestine after induction of dysbiosis in rats with antibiotics.
Animals
Male Wistar rats (200–250g, 6–7weeks of age) were obtained from the Animals Breeding Center, Rappolovo, Russia. Rats were maintained in separate cages under constant conditions at room temperature (18–22 °C), on a 12h light/dark cycle, with the noise level not exceeding 85dB, at 50–60% humidity, and were provided with free access to water and standard rat pellets (complete compound feeds for laboratory rats and mice, PK-120 sh. 1492, state industry standard R 50258-92 in pellets with diameter 14mm, Russia). This study was carried out in strict accordance with the necessary ethical requirements and in compliance with the principles of humane treatment of animals (of the European Communities No 86/609 EU). The study was approved by the local ethics committee of FSBSI «IEM».
Collection and storage of fecal samples
Fecal matter was collected from animals, stored in a freezer at -80 °C, and used for indigenous fecal transplantation (administration of rat feces after dysbiosis induction) and for preparation of anaerobic consortium (AC). Before feces were introduced, they were thawed and resuspended in a sterile phosphate buffer saline (PBS, pH 7.4, 137mmol/L NaCl, 2.7mmol/L KCl, 10mmol/L Na2HPO4, 1.8mmol/L KH2PO4)) at a ratio of 50mg per 1ml.
Anaerobic consortium cultivation conditions
The community of fecal bacteria originally taken from each individual rat was fed to the same animal. 20 mg of rat feces were thawed after storage at -80 °C, added to 10ml of thioglycolate medium (Pronadisa, Spain) together with 0.5 μg/ml Vitamin K, 100µl 10% gelatin and 150µl 40% D-glucose, thoroughly mixed, and cultivated in an anaerobic system (CO2 incubator) at 37 °C for 6days.
Rat model of antibiotic-associated dysbiosis
Experimental intestinal dysbiosis was induced in rats via daily intragastrical administration with metal tip of ampicillin ® (Orgenica, Russia) at dose of 75mg/kg and metronidazole ® (Nycomed, Denmark) at dose of 50mg/kg for three days.14,31
Design of the study
The rats were divided into two experimental and two control groups with 12 animals in each group. The experimental (A and F) and control group (C1) first received ampicillin and metronidazole for 3days for the purpose of inducing dysbiosis according to the previously published protocol,20 after which C1 group received PBS. After receiving antibiotics for 3days, the animals from experimental groups were fed with anaerobically grown fecal microbiota (group A) or indigenous feces, that were previously (before induction of dysbiosis) prepared from their own fecal samples (group F). The second control group (C2) received water instead of antibiotics and PBS. The study design is shown in Table 1. On 9th day of experiment fecal samples were taken from rats to analyze the composition of intestinal microbiota. The chyme segments were taken after the necropsy of animals from various parts of the small intestine (duodenum, jejunum, ileum) and from the large intestine, and were stored at -80°C and frozen for the purpose of determining the activity of digestive enzymes of chyme.
Groups of rats |
1-3days |
3-8days |
9thday |
F |
Ampicillin+metronidazole |
Autologous feces |
Fecal samples, |
A |
Ampicillin+metronidazole |
Anaerobic consortium |
|
C1 (Control 1) |
Ampicillin+metronidazole |
Phosphate buffer saline |
|
C2 (Control 2) |
Distilled water |
Phosphate buffer saline |
Table 1 Study design
Microbiome study
The fecal samples and AC content were analyzed by 16S rRNA gene-based metagenomics analysis. Changes in the gut microbiota content were investigated by performing 16S rRNA gene-based metagenome analysis using a previously described approach.18
Biochemical analysis
Maltase (ML, EC 3.2.1.20), alkaline phosphatase (AP, EC 3.1.3.1), and aminopeptidase N (AMN, EC 3.4.11.2,) activity was determined in homogenates of chyme from different segments of the intestine using methods described earlier.20,32 Chyme samples were obtained from the small intestine, as well as from the colon. For this purpose, each section of the intestine was washed from the cavity with cold Ringer's solution (pH 7.1-7.4) 30ml. Specific activity of every enzyme was calculated as μmol/intestine segment and μmol/per 1g wet weight of mucosal membrane or chyme collected from the intestine segment.
Statistical analysis
Statistical analysis was performed using the software package Statistica 8.0. (StatSoft, USA). Differences between the groups were obtained using Kruskal–Wallis tests and ANOVA with post-hoc HSD test for unequal n, p<0.05 was considered significant.
In this study the correction of experimental dysbiosis in rats was performed using: 1) autologous (indigenous) feces taken from animals before dysbiosis induction, and 2) AC obtained by cultivating a fecal suspension under anaerobic conditions. Experimental dysbiosis was induced following a procedure described earlier.1,14,20,31 This study, as well as earlier studies,1,20,31 revealed: an increase in the representation of Proteobateria, together with a decrease in the relative abundance of Enterococcus spp., Lactobacillus spp., Prevotella spp. and Bacteroides spp. after the administration of ampicillin and metronidazole.
Effect of autoprobiotics on the microbiome
Composition of the microbiome after a 5day recovery period in groups C1, A, and F at the phylum, family and genus levels are shown in Figures 1-4.

Figure 1 Bacterial composition in the fecal samples of rats from different groups at phylum level (metagenome study).А – Group after correction of experimental intestinal dysbiosis in rats using anaerobic consortium. F – Group after correction of experimental intestinal dysbiosis in rats by autologous fecal transplantation. C1 – control group 1, without correction of experimental intestinal dysbiosis in rats. C2 – control group 2, without induction of intestinal dysbiosis. *P<0.05 when compared with C2 group, ** P<0.05 when compared with C1 group.
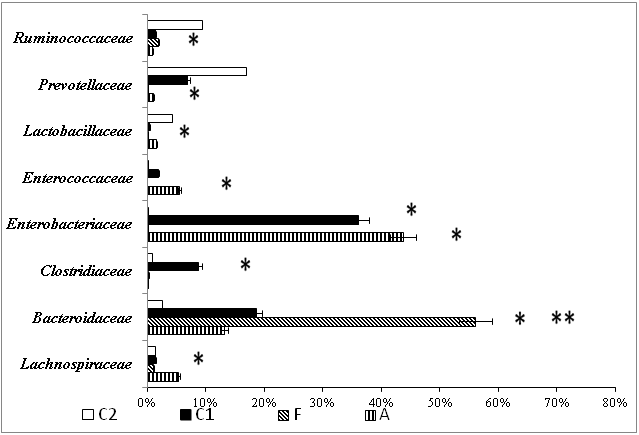
Figure 2 bacterial compositions at family level in the fecal samples of rats from different groups (metagenome study).А – Group after correction of experimental intestinal dysbiosis in rats using anaerobic consortium. F – Group after correction of experimental intestinal dysbiosis in rats by autologous fecal transplantation. C1 – control group, without correction of experimental intestinal dysbiosis in rats. C2 – control group, without induction of intestinal dysbiosis. *P<0.05 when compared with group C2, ** P<0.05 when compared with C1 group.

Figure 3 Bacterial compositions at genus level of Gammaproteobacteria in the fecal samples of rats from different groups (metagenome study). А – Group after correction of experimental intestinal dysbiosis in rats using anaerobic consortium. F – Group after correction of experimental intestinal dysbiosis in rats by autologous fecal transplantation. C1 – control group , without correction of experimental intestinal dysbiosis in rats. C2 – control group, without induction of intestinal dysbiosis. *P<0.05 when compared with group C2, ** P<0.05 when compared with group C1.
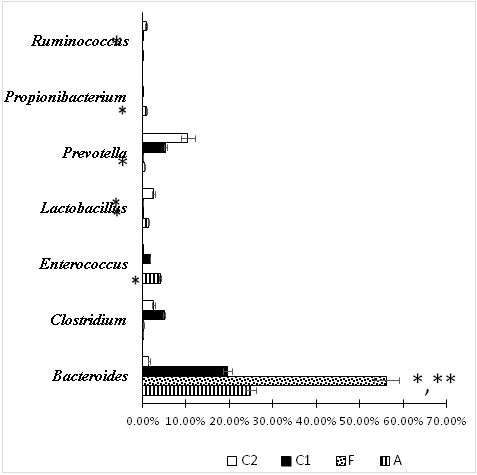
Figure 4 Composition of statistically significant genera of anaerobic bacteria and Enterococcus spp. in the fecal microbiome of rats after dysbiosis recovery. А – Group after correction of experimental intestinal dysbiosis in rats using anaerobic consortium. F – Group after correction of experimental intestinal dysbiosis in rats by autologous fecal transplantation. C1 – control group, without correction of experimental intestinal dysbiosis in rats. C2 – control group, without induction of intestinal dysbiosis. *P<0.05 when compared with group C2, ** P<0.05 when compared with group C1 .
Levels of Firmicutes remained low and Proteobacteria remained high in C1 compared to C2. The microbiome did not completely recover even after treatment with AC and AFMT (for 5 days). Bacteroidetes representation was higher in group F, than in other groups. Whereas Firmicutes and Proteobacteria representation was lower, than in groups С1 and С2. Group A had the highest representation of Firmicutes and Proteobacteria. The introduction of AC with a high representation of bacteria belonging to the phylum Firmicutes, resulted in the complete recovery of this phylum, which did not occur in groups C1 or F.
Changes of microbiota content at the family level were even more informative (Figure 2). The relative abundance of Enterobacteriaceae family was increase in groups A and C1 when compared to other groups. But after AC consumption this parameters was higher than in group C1. The share of Lactobacillaceae was lower in groups F and C1, than in groups A and С2. Enterococcaceae representation was higher in group A, than in group C2, and lower in group F than in group C1. Lachnospiraceae representation was higher in group A, than in other groups.
Changes of microbiota content at the genus level are presented in figure 3. The prevalence of opportunistic bacteria, belonging to the genera Klebsiella, Proteus, Serratia, Suterella and Serratia has been proven in group C1. An increase of relative abundance of Klebsiella spp. was observed in group A, but it was lower than in group C1. The highest relative abundance of Propionibacterium spp., Lactobacillus spp. and Enterococcus spp. was revealed after AC consumption. Representation of genus Ruminococcus tended to decrease in all groups after exposure to antimicrobial agents when compared to C2, but this decrease was statistically significant only when comparing groups C1 and C2.
The highest Clostridium spp. representation was found in C1 (it was higher than in A and F). This may be due to an increase in the population of Clostridium difficile, which we noted earlier when using a quantitative polymerase chain reaction.1,20 It was noteworthy that Group F had the highest Bacteroides spp. relative abundance more than in all other groups. Representation of this genus in other groups did not differ from the C2 group.
The inverse dependence of the bacterial content of these two genera, apparently, was not accidental. It was also noted by other authors in the study of the microbiome of humans and animals.33–35 Correlation analysis revealed that relative abundance of Roseburia spp. and Bacteroides/Prevotella inversely correlated with the parameters of host metabolism.35 In our studies, a comparison of the representation of different bacteria also revealed an inverse correlation between representatives of Bacteroides and Prevotella genera and related families (Figure 5). It cannot be excluded, that these bacteria compete with each other by engaging in metabolic reactions that occur in the organism in close interaction between eukaryotic cells and microorganisms. The microbiome of rats after AC and AMFT administration for correction of dysbiosis and animals from the control group C1 (natural recovery) and control group C2 (where dysbiosis was not induced) are compared in Table 2.
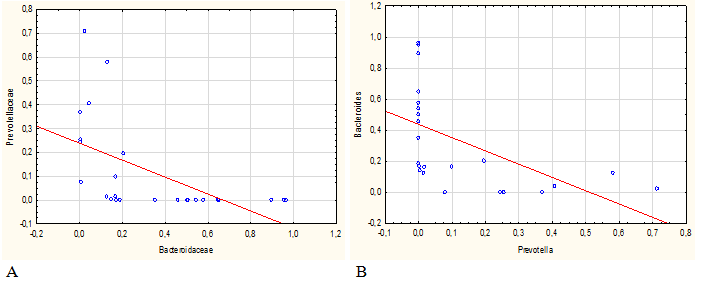
Figure 5 Direct and inverse correlation between the content of Prevotella spp. and Bacteroides spp. at the level of families (A, R=0,85) and genera (B, R=0,85).
Changes in microbiome |
|
С1 |
F |
A |
More than in control group 2 (healthy rats) |
Philum |
Proteobacterium |
Bacteroidetеs, |
Proteobacteria |
Family |
Enterobateriaceae |
Bacterioidaceae, |
Enterobacteriaceae, |
|
Genus |
Proteus |
Bacteroides |
Klebsiella, |
|
Less than in control group 2 (healthy rats) |
Phylum |
|
Firmicutes |
|
Family |
Lactobacillaceae, Ruminococcaceae |
Prevotellaceae |
Prevotellacae |
|
Genus |
Lactobacillus, Ruminococcus |
Enterococcus Lactobacillus |
|
Table 2 The feature of recovery intestinal microbiome of rats from different groups at the phylum, family and genus levels. (Summary results)Note: consumption after induction of dysbiosis С1 – phosphate buffer saline; F- autologous microbial fecal transplant; A- autoprobiotic consortium.
Activity of digestive enzymes in the intestine
In order to detect changes in digestive physiology and its recovery using AC and AFMT, we considered key enzymes involved in the final stages of hydrolysis of phosphoric acid esters (alkaline phosphatase, AP), carbohydrates (maltase, ML) and proteins (aminopeptidase N, APN) in the chyme of various enteral segments. Compensatory activity has been previously studied on probiotic strains and their combinations. This activity was both general and specific to individual strains and their mixture.20,32
Activity of intestinal alkaline phosphatase
AP activity in chyme in proximal parts of small intestine of rats from group C1 decreased sharply as opposed to other groups (Table 3). This fact has proven to be the least favorable because AP have been implicated in mediating host-bacterial interactions through their ability to dephosphorylate lipid A, component of lipopolysaccharide (LPS), the endotoxin of Gram-negative.36,37
Group |
Duodenum |
Jejunum |
Ileum |
Colon |
Control 2 |
31.03±4.99 |
17.97±1.99 |
11.03±2.37 |
2.84±0.73 |
Control 1 |
15.59±3.48* |
2.29±3.32* |
6.35±1.46 |
2.29±0.29 |
F |
19.31±1.80 |
17.52±1.90 |
6.64±0.76 |
1.85±0.26 |
A |
20.73±1.76 |
14.48±1.15 |
6.93±0.51 |
4.98±0.91*,** |
Table 3 Activity of alkaline phosphatase in the chyme of rats from different groupsNote: А – group after correction of experimental intestinal dysbiosis in rats using anaerobic consortium. F – group after correction of experimental intestinal dysbiosis in rats using autologous fecal transplantation. C1 – control group 1, without correction of experimental intestinal dysbiosis in rats. C2 – control group 2, without induction of intestinal dysbiosis. *P<0.05 when compared with group C2, ** P<0.05 when compared with group C1.
Practically full recovery of AP activity in the upper small intestine chyme occurred in groups A and F. The increase in AP in the proximal sections can be seen as a compensatory reaction to inflammation and protection of the liver from LPS. We have already observed this phenomenon with «low-grade inflammation» when probiotic strain Enterococcus faecium L3 was used for the experimental dysbiosis correction.9
AP activity analyses revealed that in the colon of group A animals AP was notably increased. This activity was significantly higher in comparison with groups C1 and F and tended to increase in comparison with intact rats (group C2). High AP activity in the distal region of the intestine after AC exposure may be related with the increase of Lactobacillus spp., Enterococcus spp., the members of Enterobacteriaceae family, that are capable of producing this enzyme.36.38 Coprococcus spp. and Dorea spp., part of the Lachnospiraceae family, the representation of which was increased in groups A, could also have affected AP activity by acting on the microbiota and enzyme action of intestine cells.
It has also been demonstrated that AP activity decreased after large quantities of Bacteroides spp., including heat-killed bacteria, were administered to mice.39,40 This trend was not confirmed in our experiment. The low AP values in the large intestine chyme of group F animals can be not linked to high Bacteroides spp. content in gut microbiota after exposure to indigenous feces. It cannot be excluded that this was due to the species characteristics of the Bacteroides spp., which have a variety, manifesting themselves in the biochemical properties.26,33
Activity of intestinal maltase
Maltase (ML) is a key enzyme in breaking down the most common carbohydrate biopolymer – starch.41 Previously it has been demonstrated, that the administration of ampicillin and metronidazole led to decrease in the activity of ML in the chyme.32 Whereas exposure to probiotic enterococci in correction of dysbiosis reduced the activity of ML in the mucous membrane of the small intestine, apparently to compensate for an increase in the activity of this enzyme in the chime.14 The ML activity in the large intestine of all rats from A, F and C1 was decreased (Table 4). We have also found a significant increase of ML activity in the chyme of jejunum and ileum in group F. It is possible, in the case of an increase of Bacteroides spp. and decreased of Prevotella spp. in the small intestine of rats, which received AMFT. Bacteroides is a metabolically active bacterial group that is involved in polysaccharide degradation.42 This assumption is supported by the revealed correlation (Figure 7).
Group |
Duodenum |
Jejunum |
Ileum |
Colon |
Control 2 |
39.36±6.59 |
23.14±2.93 |
30.43±2.33 |
10.42±2.55 |
Control 1 |
29.52±4.10 |
19.99±3.54 |
27.65 1.73 |
6.03±0.56* |
F |
34.71±.68 |
32.08±3.84 ** |
35.91±2.86** |
4.69±0.82* |
A |
34.94±1.76 |
23.83±1.36 |
25.84±1.59 |
5.20±0.48* |
Table 4 Activity of maltase in the chyme of rats from different groupsNote: А – group after correction of experimental intestinal dysbiosis in rats using anaerobic consortium. F – Group after correction of experimental intestinal dysbiosis in rats using autologous fecal transplantation. C1 – control group 1, without correction of experimental intestinal dysbiosis in rats. C2 – control group 2, without induction of intestinal dysbiosis. *P<0.05 when compared with group C2, ** P<0.05 when compared with group C1.
Correlation analysis has demonstrated that there is a direct correlation between Bacteroides representation and maltase activity in small intestine chyme and an inverse correlation for Prevotella (Figure 6).
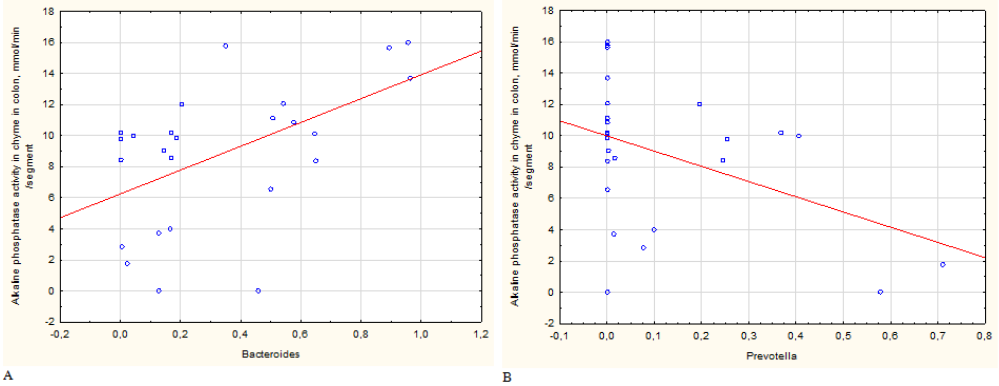
Figure 6 Significant correlation between maltase activity in chyme and a relative abundances of members of genera Bacteroides (A, R=0,53) and Prevotella (B, R=-0,46).
Activity of aminopeptidase- N
APN is involved in the hydrolysis of oligopeptides, in the cholesterol transport across intestinal epithelium43 in immune44 and inflammatory responses.45 We have demonstrated earlier that enzyme activity in chyme from the small and large intestine was higher after induction of dysbiosis than in control group.32 APN activity was compensated by using probiotic Lactobacillus fermentum Z and E. faecium L3, but was not compensated by probiotic Escherichia coli M17 consumption.
АPN activity in chyme of large intestine was higher than in control group C2 in all groups (Table 5). Considering reports by other authors that pepsidase inhibitors, including АPN, improve colitis in mice.45 It can be suggested that the increase in this enzyme activity in the large intestine in groups C1 and A could be related, at least partly, to inflammation in the intestine. Inflammation in group C1 could be related to the increase population of Clostridium spp., Klebsiella spp., Proteus spp., Serratia spp. and Suterella spp. It could be connected with the increase of Proteobacteria and in particular Klebsiella spp. relative abundance in the case of group A. The presence of increased activity of the enzyme in the distal intestines in group F may be associated with an increase in the population of Bacteroides spp. which possess proteolytic activity and are referred to as «proteolytic bacteria».26The decrease in activity of APN in the chyme from the duodenum and jejunum only in rats from group F remains difficult to explain. The total results of the experiment are presented in Table 6.
Group |
Duodenum |
Jejunum |
Ileum |
Colon |
Control 2 |
0.201±0.051 |
0.843±0.020 |
1.18±0.25 |
0.033±0.011 |
Control 1 |
0.130±0.026 |
0.70±0.11 |
0.80±0.10 |
0.24±0.11* |
F |
0.087±0.014* |
0.495±0.072* |
0.896±0.035 |
0.128±0.018* |
A |
0.127±0.021 |
0.658±0.045 |
1.01±0.14 |
0.36±0.11* |
Table 5 Activity of aminopeptidase N in the chyme of rats from different groups Note: А – group after correction of experimental intestinal dysbiosis in rats using anaerobic consortium. F – Group after correction of experimental intestinal dysbiosis in rats using autologous fecal transplantation. C1 – control group 1, without correction of experimental intestinal dysbiosis in rats. C2 – control group 2, without induction of intestinal dysbiosis. *P<0.05 when compared with group C2, **P<0.05 when compared with group C1.
Enzymes |
Parts of intestine |
C1. |
F |
A |
AP |
Duodenum |
< C2 |
|
|
Jejunum |
<C2 |
|
|
|
Ileum |
|
|
|
|
Colon |
|
|
>С2 |
|
Maltase |
Duodenum |
|
|
|
Jejunum |
|
>C2 |
|
|
Ileum |
|
>C2 |
|
|
Colon |
<C2 |
<C2 |
<C2 |
|
AMP N |
Duodenum |
|
<C2 |
|
Jejunum |
|
<C2 |
|
|
Ileum |
|
|
|
|
Colon |
>C2 |
>C2 |
>C2 |
Table 6 Summary results. The levels of recovery microbiome and activity of digestive enzymes
It is clear that complete recovery of enzyme activity so as intestinal microbiota composition was not revealed at the end of experiments. The changes were general and specific. This was characteristic of a decrease in maltase activity and an increase in APN in colon of all animals with intestinal dysbiosis despite of specific consumption after antibiotic introduction. The changes in microbiota and digestive function can be considered as adaptation during recovery period after «low grade inflammation» accompanied by excessive growth of opportunistic enterobacteria and a decrease of relative abundance of resident of the microbiota representatives. This inflammation could be expressed to a greater extent in the group in which the correction of dysbiosis was not performed.
This study allowed comparison of key digestive enzyme activities in different parts of the intestine in parallel with the study of microbiome. The intestinal microbiome was evaluated by fecal samples characterizing whole microbiota, with an emphasis on distal parts of the gastrointestinal tract. In perspective it is important, to look at the effect on the settlement of microorganisms in different parts of the intestine in case of dysbiotic disorders. The most interesting are the results of comparison of the effects of AC, native bacteria grown under anaerobic conditions under which lactobacilli and enterococci obtained selective advantages and AMFT.
When AC were injected, we risked additional suppression of normal microbiota representatives described after probiotic lactobacilli administration to experimental animals and humans after exposure to antibiotics.6,7 Suez and co-authors invasively examined the effects of multi-strain probiotics or AFMT on post-antibiotic reconstitution of the murine and human mucosal microbiome niche and found that Lactobacillus was a microbiome-inhibitory species.
However, the presence of indigenous lactobacilli and other indigenous bacteria allowed not only to restore the population of lactobacilli, but also to increase colonization resistance to a number of opportunistic bacteria, increasing the representation of resident representatives of normal intestinal microbiota (Lachnospira spp., Propionibacterium spp. and Escherichia coli). The latter were detected earlier in a bacteriological study and by quantitative PCR.18,46 Against the background of administration of AC, dysbiosis associated with excessive representation of opportunistic bacteria was associated only with genus Klebsiella, which representation was fewer than in the C1 group. A feature of metabolic changes in A group compared to healthy rats was an increase of AP activity in the distal parts of gastrointestinal tract, which could be associated with an increase of this enzyme producers relative abundancе. The main producers of AP in the colon are enterococci and enterobacteria.36
On the other hand, the action of AMFT was associated with a sharp increase in Bacteroides spp. content. It cannot be ruled out that this was precisely what led to the increase of maltase activity in the small intestine rats from group F. These changes in maltase activity correlated with an increase in the representation of Bacteroides spp. and a decrease in the Prevotella population. In addition, in this group of rats, there was a decrease not only in Prevotella, but also in lactobacilli and enterococci. The reason for the decrease in APN activity in the proximal small intestine of rats from this group remained unclear.
It is impossible to exclude the direct and indirect effect of introduced indigenous bacteria on signaling as a trigger for altering of digestive enzymes expression, absorption and functioning of transporters and intestinal evacuation ability, which was previously described in a study of the effect of probiotics on digestion.30,47 The correlations discovered in this pilot study revealed some functions and mechanisms of action of individual populations of microbiota, in particular Bacteroides and Prevotella as they affect metabolic processes.
In this study, we developed a model of experimental dysbiosis in Wistar rats and studied the effect of autologous fecal transplantat (AMFT) and an anaerobic consortium and Delzene (AC) for the correction of intestinal microbiota and digestion. The discovered features of spontaneous recovery of intestinal microbiota, of the effect of AMFT and AC on the organism, its microbiome, and digestive system demonstrate the fundamental differences in the ways homeostasis correction.
Despite more pronounced residual changes in intestinal microbiome of rats from group C1, no significant changes in the activity of key digestive enzymes were detected. AMFT induced more sufficient changes in the carbohydrate and protein metabolism (the increased ML activity in small intestine and reduced APN activity in the chyme in the virtually throughout the intestine), which can be connected primarily with an excessively large population of bacteroides. AC almost completely compensated for the dysbiotic disorders due to the increase in the population of non-pathogenic enterobacteria and a number of resident bacteria, which caused a specific increase in AP activity in the distal gastrointestinal tract section in this group of animals. It is necessary to take into account the individual characteristics of the digestive system of organism and its microbiome and the possible specific influence of autoprobiotics (AMFT and AC) on it, when choosing a method for the correction of dysbiosis disorders.
None.
Author declares that there are no conflicts of interest
This work was supported by the Russian Science Foundation №16-15-10085.

©2020 Ermolenko, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.