eISSN: 2373-6372


Case Report Volume 9 Issue 4
1Professor of Pathology, MES Medical College, India
2Junior Resident, MES Medical College, India
3Assistant Professor, Gastroenterology, MES Medical College, India
Correspondence: Jayalakshmy PS, Professor of Pathology, MES Medical College, ?Parijatham? Royal Avenue, Kuttur.p.o, Thrissur-680013, Kerala, India
Received: May 30, 2018 | Published: July 13, 2018
Citation: Jayalakshmy PS, Rajan R, Sideeque A, et al. Synchronous occurrence of gastro-intestinal stromal tumour of small intestine and anorectal adenocarcinoma with recurrence and metastasis. Gastroenterol Hepatol Open Access. 2018;9(4):134-137. DOI: 10.15406/ghoa.2018.09.00311
The simultaneous diagnosis of gastrointestinal stromal tumour of the gastro intestinal tract and gastro intestinal adenocarcinoma has been reported many times in the medical literature with various site combinations. We report a case of high risk gastrointestinal stromal tumour of small intestine and polypoid anorectal adenocarcinoma. The uniqueness of this case is that the anorectal adenocarcinoma recurred as a frankly invasive ulceroproliferative growth and the gastrointestinal stromal tumour metastasized to both lungs, liver and bone.
Keywords: ano-rectal adenocarcinoma, gastrointestinal stromal tumour GIST, synchronous, recurrence, metastasis
GIST, gastro intestinal stromal tumour; GIT, gastro intestional tract; CT, computed Tomography
Gastro intestinal stromal tumour (GIST) is a mesenchymal neoplasm of the gastro intestinal tract (GIT). GIST represents 1% of all gastrointestinal neoplasms. Most common location is the stomach. Small intestinal GISTS comprises 30% of the GISTs of GIT. Occurrence of GIST with other primary adenocarcinomas in the gastro intestinal tract has been reported. Most common combination is GIST of stomach with colorectal adenocarcinoma and many cases have been reported. Synchronous occurrence of small intestinal (ileal) GIST with anorectal adenocarcinoma is rare. GISTs are seen in extra intestinal location like mesentery, peritoneum, retroperitoneum and other sites and are designated as Extra Gastro Intestinal Stromal tumour (EGIST). We report a case of a 51year old male patient with synchronous presentation of ileal GIST and polypoid anorectal adenocarcinoma and later with recurrence of the colorectal carcinoma and metastasis of GIST to liver, lungs and bone.
A 51year old male patient presented with complaints of heaviness of abdomen and incessant hiccups of 3weeks duration. No relevant family history. He is a known diabetic since 12years, on treatment. Per abdomen examination showed a globular Intra-abdominal mass, non tender with restricted mobility. Plain and contrast Computed Tomography (CT) study showed a large exophytic mass attached to the ileum located in the anti mesenteric border with adhesion to the sigmoid colon. Another smaller mass was seen projecting from the antimesenteric border of the ileal segment. All other organs- liver, spleen, both kidney, pancreas, gall bladder were normal by CT. No intra abdominal lymph nodes detected (Figure 1A) (Figure 1B).
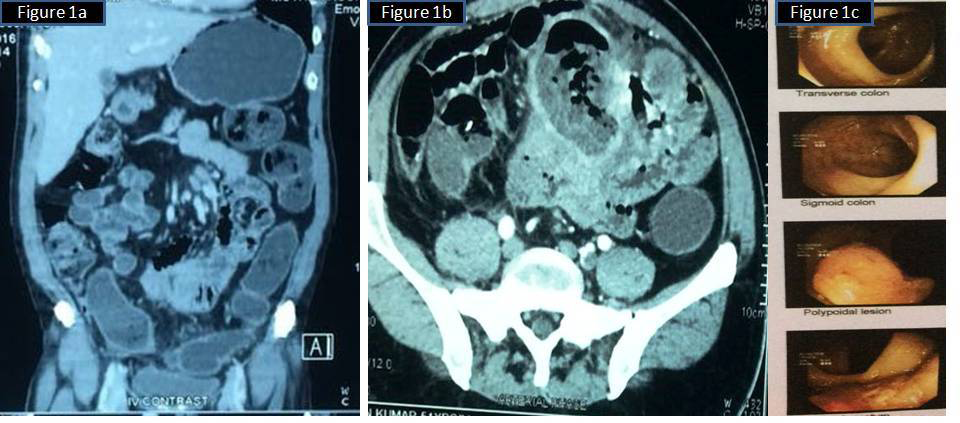
Figure 1A,1B CT abdomen showing large necrotic mass attached to small intestine 1C Colonoscopy showing a polypoid lesion 5cms above the anal verge.
Per rectal examination and colonoscopy showed a 3x2cm polyp in the rectum located 5cms from the anal verge (Figure 1C). No other polyps were identified. Rest of the intestine upto ileocaecal junction was normal in colonoscopy. Laparotomy showed 2 masses attached to the wall of the small intestine, larger in the mesenteric border of the ileum with necrotic centre and adhesions to small bowel and sigmoid colon, smaller in serosa in the anti mesenteric border. Patient underwent total excision of intra-abdominal bowel related mass with the affected area of the small intestine, with a segment of adherent sigmoid colon. Trans-anal excision of ano-rectal polypoid mass was also done from a local hospital (Figure 2A–2C). There was no tumour deposits in the peritoneal cavity or other intra abdominal organs.
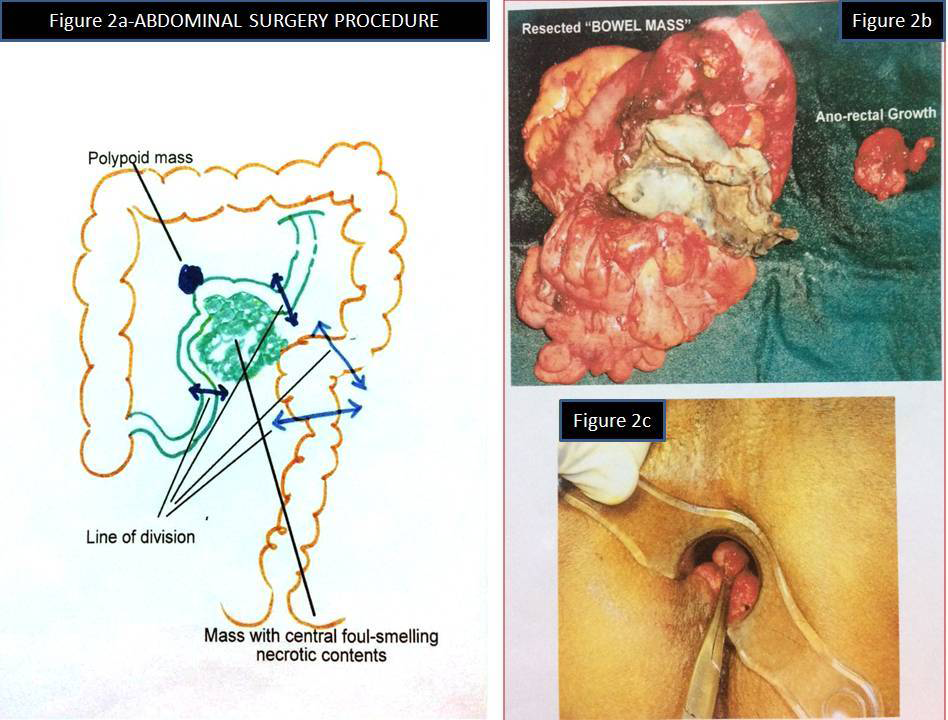
Figure 2A. Abdominal surgical procedure (Surgeon’s drawing)
B. Resected bowel mass & anorectal mass
C. Proctoscopic view of rectal polyp.
Grossly, the larger tumor with central cystic degeneration in the small intestine (Ileum) was attached to its wall and adherent to the serosal adipose tissue of the sigmoid colon. The mass measured 10.5x9.5cms. The smaller mass measured 5.5x4.5cms and seen attached to the serosa of the small bowel. Microscopically, both the tumour showed-spindle cells and epithelioid cells arranged in varying patterns. Spindle cells showed long and short fascicular, cellular, oedematous, palisaded , whirling and other pattern. Epithelioid component was arranged in nested and morular pattern. Mitosis was more than 5/50HPF. Areas of necrosis, hemorrhage and ulceration were present. Both bowel masses were reported as high risk (malignant) GIST (Figure 3A–3I). Rectal polyp was reported as well differentiated adenocarcinoma arising in a high grade tubulo-villous adenoma (Figure 4A–4D). Patient was not willing to take further treatment and was lost follow up in the local hospital.
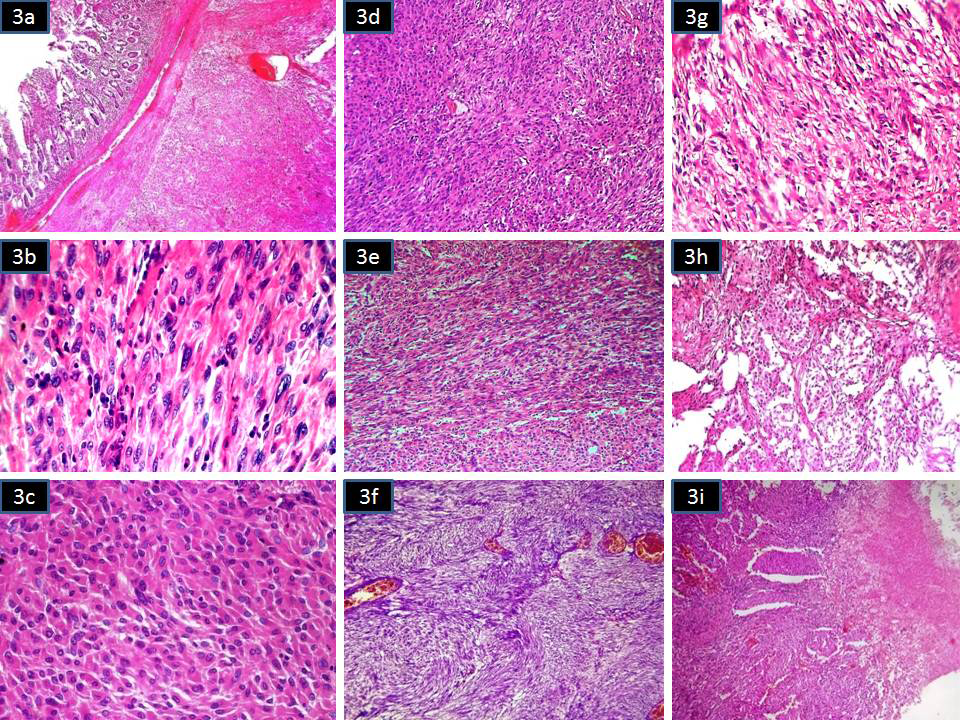
Figure 3Microscopy of GIST with spindle cell and epithelioid cells arranged in varying patterns
3A Tumour in the submucosa of the small intestine (H&Ex40)
3B Spindle cell area with collaginisation (H&Ex400)
3C Epithelioid area (H&Ex400)
3D Cellular spindle cell area (H&Ex100)
3E Long fascicles of spindle cells (H&Ex100)
3F Palisaded area of spinle cells (H&Ex100)
3G Oedematous area (H&Ex100)
3H Nested pattern of epithelioid cells (H&Ex100)
3I Tumour cells with areas of necrosis (H&Ex100).
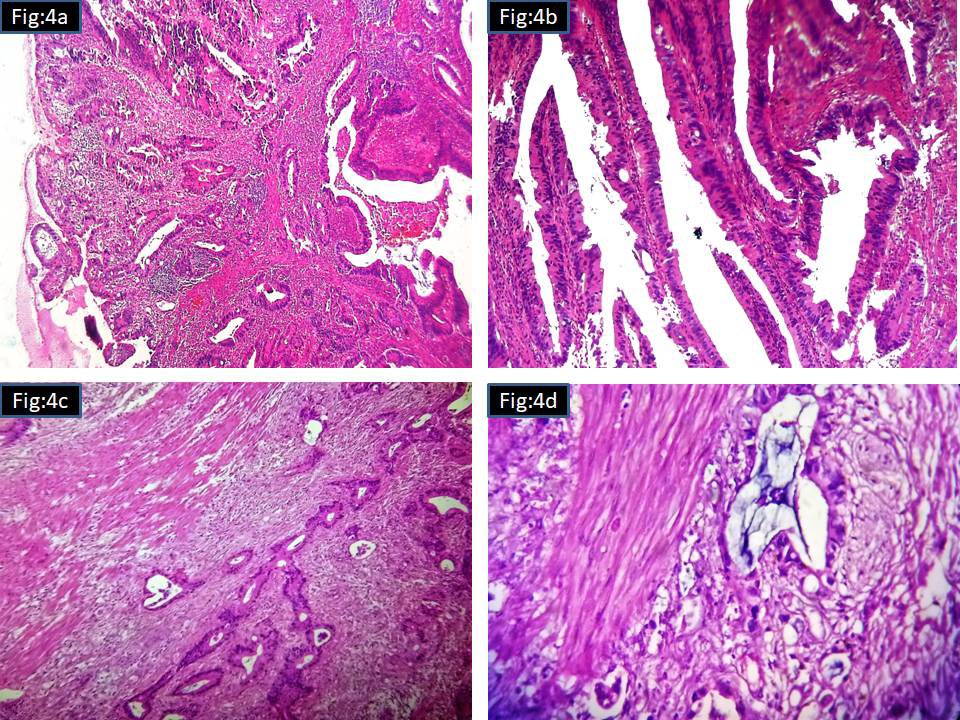
Figure 4 Microscopy of rectal polyp
4A Scanner view (H&Ex40)
4B Tubulo villous pattern with high grade dysplasia (H&Ex400)
4C Stalk invasion (H&Ex100)
4D Invasive tumour (H&Ex400).
After 1year 9months, patient presented in our hospital, a tertiary care centre, with complaints of altered bowel habits of 4 months duration. At this time, colonoscopy showed an ulcero-proliferative growth in the rectum starting from the anal verge and extending up to 10 cms. A polyp was also noted 1x1cm, in the rectum 19cms from the anal verge (Figure 5A) (Figure 5B). MRI of the liver showed multiple well defined enhancing lesions in both lobes largest 3.1x2.3cms. Lung showed small nodules diffusely scattered in both lung parenchyma (Figure 6A) (Figure 6B). Mestastatic deposits were also noted in both iliac bones.
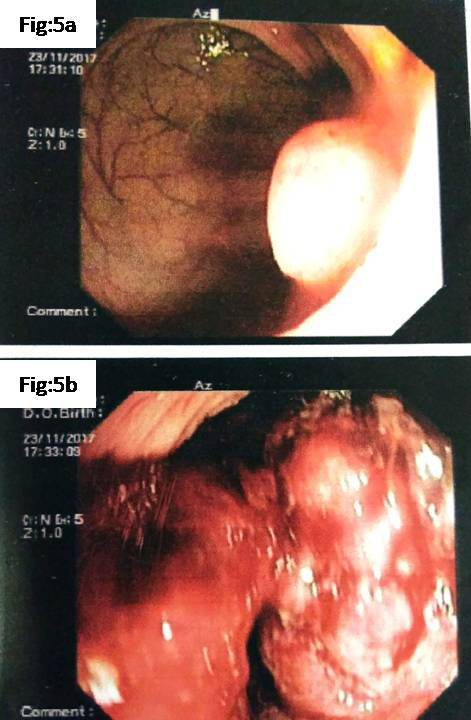
Figure 5 Present colonoscopy
A. 5A Small polyp located 19cms from anal verge
B. 5B Ulceroproliferative growth in the rectum starting from anal verge extending up to 10 cms.
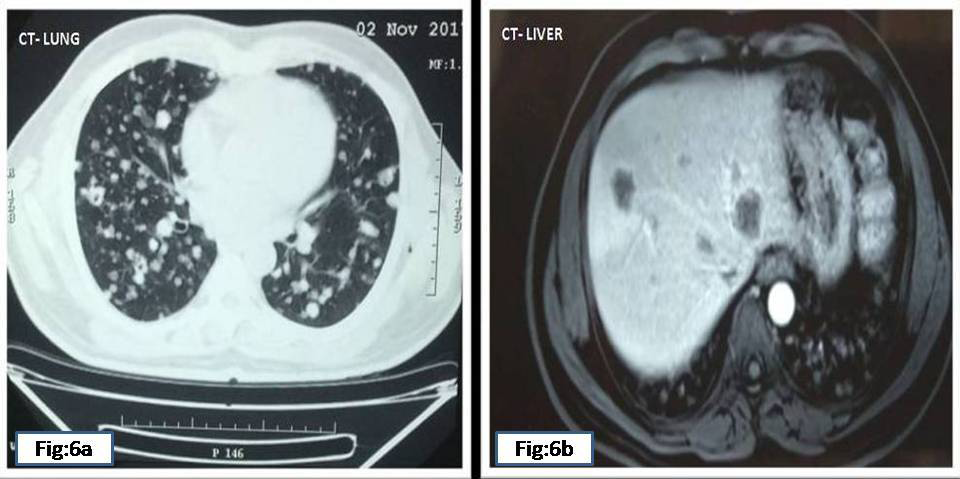
Figure 6 Present CT
6A Bilateral multiple metstatic deposits in lung
6B Multiple metastatic deposits in both lobes of liver.
Biopsy from the ulcero proliferative growth in the rectum and lesion in the liver was taken. The rectal growth was reported as moderately differentiated adenocarcinoma (Figure 7A) (Figure 7B). Liver biopsy showed a malignant spindle cell lesion which was confirmed to be GIST by Immunohistochemistry (Figure 8A–F). The patient now has a recurrent colonic adenocarcinoma and multiple metastatic GIST in liver, both lungs and bone. The patient is on chemotherapy and symptomatically better. But the lesions in the lungs and liver has not completely cleared.
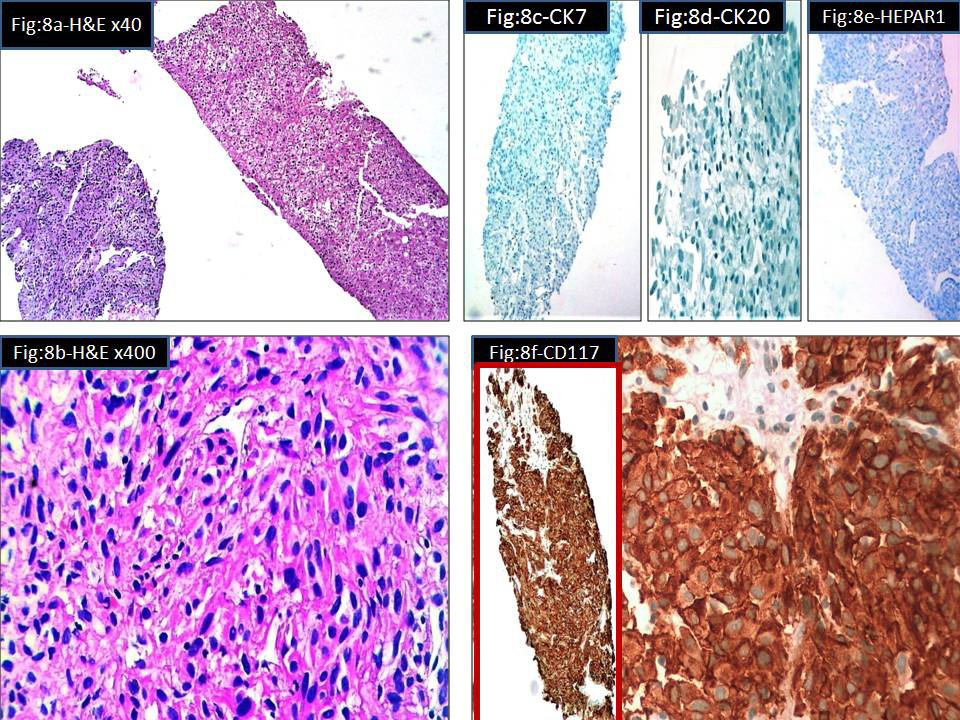
Figure 8 Microscopy of liver biopsy showing metastatic GIST
8A Liver tissue (right side) & tumour tissue (left side) (H&E x40)
8B Tumour composed of sheets of spindle cells (H&E x400)
8C Immunostain for CK 7-negative
8D Immunostain for CK20-negative
8E Immunostain for Hepar 1-negative
8F Immunostain for CD117(c-Kit) Strong diffuse positivity in tumour cells.
GISTs arise most commonly in the stomach (60%) and jejunum and ileum (30%) and less frequently in the duodenum (5%), colorectum (<5%), and esophagus (<1%).1 Most are sporadic. 10% associated with syndromes like Carney’s triad, Neurofibromatosis1, Carney-Stratakis syndrome and others. One of the authors of this article has reported a case with a rare combination of ileal GIST and pheochromocytoma in a neurofibromatosis 1patient.2 There is an increasing number of literature reports on synchronous occurrence of gastrointestinal stromal tumors and another malignancy of distinct etiology and evolution.3 GIST arises from interstitial cells of Cajal and adenocarcinoma is an epithelial malignancy. Both are histogenetically different. Majority of the GISTs are demonstrated to have mutation in either c-kit or PDGFRA. But none of the colonic cancer is demonstrated to have mutations in these genes. Hence by the molecular genetic pathway also the two are not related. So, why and how such a combination is occurring in certain patients is a subject for further research. A minority of the GISTs carries BRAF mutation and whether this is somehow related to the development of synchronous gastro intestinal carcinomas is still a matter of debate. Melis et al points out that the limited number of these cases cannot confirm the existence of a common factor in tumorigenesis of these histopathologically completely different tumors and further studies are needed toclarify the possible association.4
The simultaneous occurrence of GIST and adenocarcinoma is uncommon in the literature, often the first one being detected incidentally at surgery.5 But in our case the main complaint was the abdominal discomfort symptoms due to the GIST.Polypoid anorectalcarcinoma was detected incidentally by colonoscopy during investivations for the GIST surgery. Microscopically, most of the GISTs consist of spindle-shaped cells (70%), but some GISTs consist of rounded cells (epithelioid type, 20%) or a mixture, but they can also be pleomorphic.6 The Armed Forces Institute of Pathology (AFIP) series of 1,765 gastric GISTs, the largest single site series to date, described eight distinctive histologic variants (four spindled and four epithelioid) with consistent and reproducible morphology.7 The present case also showed a mixture of spindle cells and epithelioid cells in varying patterns of arrangement as cellular, long and short fascicles, collagenous, nested, morular and other patterns.
GIST in our patient was more than 10cms in size with more than 5mitosis/50HPF coming under the category of high risk GIST in both NIH 2001 Consensus classification scheme and in the Armed Forces Institute of Pathology risk stratification scheme. The malignant potential of high risk GIST in this patient was proved later by the metastatic deposits in multiple organs. Approximately, 20% to 25% of gastric GISTs and 40% to 50% of small intestinal GISTs behave in a clinically malignant fashion.8 Our case of small intestinal GIST was proved malignant because of metastasis to multiple organs. Surgery has so far been the only effective treatment for GIST.9 The present case had undergone surgical resection of the GISTs. The vast majority of GISTs (80%) are associated with mutations of the proto-oncogene c-Kit, a growth factor receptor of the tyrosine kinase subclass III family, normally expressed in a variety of human tissues including the interstitial cells of Cajal. GISTs expressing CD117 may respond to imatinib mesylate therapy which is a selective tyrosine kinase inhibitor.
The present case is having a combination of epithelial malignancy at one site (colorectal adenocarcinoma) and a mesenchymal malignancy at another site (small intestinal GIST) of the gastro intestinal tract simultaneously with recurrence of epithelial malignancy and metastasis of mesenchymal malignancy to multiple sites. Since synchronous occurrence of gastrointestinal stromal tumors and another malignancy is well established, thorough screening of cases with GIST for other malignancies should be promptly done in the preoperative or peroperative period. And after the initial treatment, patient should be closely followed up because of the high chance of recurrence and or metastasis of the epithelial and or mesenchymal malignancy. Our patient might have developed recurrence and malignant change because of the lack of proper treatment after initial surgery.
We, the authors of this article sincerely thank Dr. K. Kuruvila M S, Senior consultant Gastrointestinal and Laparoscopic Surgeon, Mother Hospital, Thrissur, Kerala, India for providing us the photograph of initial surgical procedure drawing (Figure 2A), the initial gross specimen of GIST, Ano rectal polyp (Figure 2B) and the proctoscopic view of the Ano rectal polyp ( Figure 2C).
We authors hereby declare that there is no conflict of interest in publishing this case.

©2018 Jayalakshmy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.