eISSN: 2373-6372


Short Communication Volume 9 Issue 2
Department of Internal Medicine/Gastroenterology Wayne State University School of Medicine, USA
Correspondence: Paul Naylor, Department of Internal Medicine/Gastroenterology Wayne State University School of Medicine, USA
Received: February 11, 2018 | Published: April 16, 2018
Citation: Naylor P, Tama M, Goyal S, et al. Racial disparity in primary biliary cholangitis (pbc) patients seen in an urban academic gi clinic. Gastroenterol Hepatol Open Access. 2018;9(2):89-90. DOI: 10.15406/ghoa.2018.09.00301
Primary Biliary Cholangitis (PBC) is a rare autoimmune disease which occurs predominantly in middle-aged Caucasian females. It causes the destruction of intrahepatic bile ducts leading to prolonged cholestasis and eventually to cirrhosis. The age-adjusted incidence of PBC in the United States is 45 women and 7 men per 1 million-person years.1–3 The prevalence of PBC is 654 women and 121 men for 1 million persons. As reported by Lau et al., the prevalence as defined by a nationwide cohort of patients in the FOLD consortium, increased from 21.7 to 39.7 per 100,000 persons between 2006 and 2014.1 The incidence reported was similar during the same time period (37 vs 34 per 1 million) to that reported in other studies. It is well known that the severity of autoimmune diseases is genetically linked and can be variable between Caucasian and African Americans (AA). With respect to prevalence and incidence, both are lower in AA compared to Caucasians.1,4 Among patients with confirmed PBC, AA were more likely than Caucasians to have severe pruritus and a more severe liver disease defined by encephalopathy, ascites or variceal bleeding based on the small number of 21 African American patients out of the total 533 patients.4
The only approved treatment options for PBC are ursodeoxycholic acid (UDCA) as first line and obeticholic acid in patients who failed or cannot tolerate UDCA.5–9 It is reported that UDCA efficacy is significantly better in the early stages of the disease as compared to patients who are started later. Identifying patients whose disease is not responsive early in the treatment to UDCA is also important so that second line therapy can be initiated in a timely manner. The goal of this study was to evaluate the racial differences in severity of PBC by identifying patients with PBC in a population that is predominantly African American. A secondary goal was to identify patients for newer second-line therapies like obeticholic acid.
ICD-9 codes were used to identify patients with an established diagnosis of PBC followed at the Wayne State University Physician Group Gastroenterology clinic in an urban academic medical center. After review of the medical records, 6 AA patients and 6 non-AA patients (5 Caucasians and 1 Hispanic) who had repeat visits were identified. A database was generated that included age, gender, race, lab tests, anti-mitochondrial antibody titers (AMA), imaging, biopsy, treatment status and comorbidities.
The PBC patients were all females and the average age at diagnosis was 61 years for both AA (range 54-73) and non-AA (range 42-78). One patient in each group had non-detectable AMA. At the first office visit available in our electronic health records, alkaline phosphatase (ALP), inflammation as defined by ALT and fibrosis as defined by APRI (p<0.04) were higher in AA patients as compared to non-AA patients (Table 1). Cirrhosis diagnosed on imaging (Ultrasound, MRI/MRCP) and/or liver biopsy was more frequent in AA patients as compared to non-AA patients (67% vs 17%).
AA |
(SEM) |
Non-AA |
(SEM) |
||
ALP |
Pre |
425 |
-148 |
252 |
-66 |
Post |
286 |
-96 |
173 |
-23 |
|
APRI |
Pre |
1.08 |
-0.31 |
0.47 |
-0.04 |
Post |
1.03 |
-0.46 |
0.31 |
-0.04 |
|
ALT |
Pre |
87 |
-27 |
61 |
-15 |
Post |
64 |
-20 |
47 |
-15 |
|
AST |
Pre |
82 |
-14 |
46 |
-6 |
Post |
52 |
-17 |
35 |
-6 |
|
Platelets |
Pre |
238 |
-40 |
266 |
-36 |
Post |
214 |
-59 |
303 |
-28 |
|
AST/ALT |
Pre |
1.09 |
-0.11 |
0.92 |
-0.15 |
Post |
0.96 |
-0.56 |
0.85 |
-0.45 |
Table 1 Racial Diversity in PBC at Earliest Visit and Response to URSO Treatment
All of the patients were treated with UDCA and the mean time on treatment was 6 years. As shown in Table 1, on average, relevant laboratory parameters improved with treatment in both groups. Figure 1 presents the pre- and post-treatment data for individual patients. At the earliest visit, the ALP value was 1.6x the upper limit of normal for 5 of the 6 AA patients but in non-AA patients it was found to be more than 1.6x ULN only in 2 of the 6. On treatment, ALP declined in 4 out of 5 AA patients with significantly elevated levels and in 1 of the two non-AA patient with significantly elevated levels. All patients had elevated (ALT) as defined by the current guidelines of 30 IU/ml for females. Response to treatment was highly variable and not correlated with ALP. AA were more likely to have significant fibrosis (APRI>0.7) than non-AA and fibrosis improvement by APRI was noted in the majority of patients with the exception of 2 AA patients whose levels either increased or remained elevated. AST/ALT ratio that has been associated with risk of disease progression in PBC patients, was less than 1.1 in most patients and was improved in 4 out of 6 AA and non-AA patients.
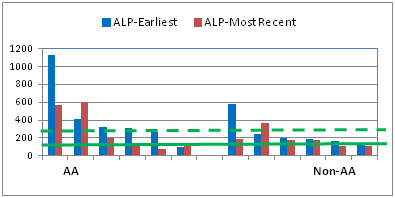
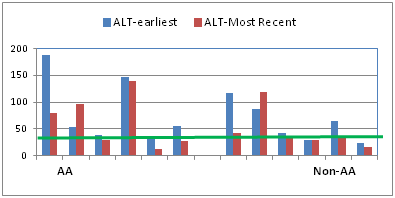

Figure 1 Individual patient response to URSO therapy by race and laboratory values. The solid lines are the upper limit of normal for ALP and ALT. The solid line for APRI represents the value for significant fibrosis. The solid line for the AST/ALP ratio represents the value which is associated with a high risk for disease progression. The dotted line for ALP is 1.6x ULN.
When assessed for possible therapy with obeticholic acid, the majority of the patients were excluded by virtue of age, comorbidities, advanced disease or the fact that the AP levels were consistently less than 1.67 times the upper limits of normal. One patient fulfilled the criteria for second line therapy and that patient was placed on therapy with subsequent improvement after 3 months in ALP (360 to 125), ALT (118 to 32) and AST (140 to 46).
The average age for diagnosis of PBC in our patients did not differ with respect to race (61years for both AA and Non-AA), but it was significantly higher as compared to the published average of 40 years for PBC patients. The reason these patients were diagnosed late in the course of the disease could be due to the socioeconomic health care challenges of urban patients and/or the failure to identify patients early in the course of their disease.
The results of this study are consistent with the reported findings by Peters et al., non-Caucasian patients (AA=21; Hispanic=42; Other=10) were less likely than Caucasian patients (n=462) to be eligible for their clinical trial due to advanced disease.4 This study confirms that non-Caucasian as compared to Caucasian PBC patients are more likely to have elevated ALP, ALT, fibrosis, and cirrhosis both before and during treatment with UDCA. AA also tend to have more severe disease with worse prognosis. Underlying mechanisms for the severe disease in AA vs Caucasians who have similar age of diagnosis is not clear at this time. The majority of patients in this study were not considered as candidates for second-line therapy due to advanced disease, age and comorbidities.
The conclusions of this study are limited due to the rarity of the disease and subsequently by the small number of patients. The study also is limited to only urban patients seen in an inner city medical center and the conclusions may not be universally applicable. The study contains a significant number of African Americans and given the rarity of the disease, conclusions based on AA patients may not be relevant to the majority of PBC patients who are non-AA.
We conclude from our study that additional efforts should be made to identify patients especially in academic urban medical centers who have PBC at an earlier time in their disease course. Perhaps screening laboratory databases for laboratory abnormalities consistent with potential PBC would be useful. Encouraging primary care physicians to screen for PBC in patients with unexplained elevations in ALP may identify candidates for appropriate therapy.
The research was supported by an Investigator Initiated Grant from Intercept Pharmaceuticals.
The author declares no conflict of interest.

©2018 Naylor, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.