eISSN: 2373-6372


Review Article Volume 7 Issue 4
1Internal Medicine Residency, Florida Hospital Orlando, USA
2Khyber Teaching Hospital, Pakistan
3Nassau University Medical Center, USA
4Section of Histopathology, Lehigh Valley Hospital Network, USA
5Eastern Pennsylvania Gastroenterology and Hepatology, USA
Correspondence: Hiral Shah, Eastern Pennsylvania Gastroenterology and Hepatology, 1501 North Cedar Crest Boulevard #110, Allentown, Pennsylvania 18104, USA, Tel 610 821 2828
Received: June 22, 2017 | Published: August 28, 2017
Citation: Asad-ur-Rahman FNU, Khan MT, Shah M, Grau J, Shah H (2017) Macroscopic Findings in Microscopic Colitis: Two Case Reports and a Review of Literature. Gastroenterol Hepatol Open Access 7(4): 00245. DOI: 10.15406/ghoa.2017.07.00245
Microscopic colitis has emerged as an increasingly common cause of chronic and debilitating watery diarrhea in the adult population. Although it was initially assumed to be a histopathologic diagnosis, recent review of literature shows specific endoscopic features that are associated with it. We report two cases of collagenous colitis that had evidence of rectal ulceration on colonoscopy. Both of these cases were seen in Caucasian females in their 60s in association with the use of selective serotonin reuptake inhibitors. This is followed by a review of literature on the subject. Further studies are needed to assess an association of medications with macroscopic findings observed in patients with microscopic colitis.
Keywords: microscopic colitis; collagenous colitis; ulceration; collagenous gastritis
MC, microscopic colitis; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; EGD, esophago-gastroduo-denoscopy; PPI, proton-pump inhibitors; NSAIDs, non steroidal anti inflammatory drugs; SSRIs, selective serotonin receptor inhibitors
Microscopic colitis (MC) is emerging as an increasingly common cause of chronic and debilitating watery diarrhea in the adult population. The term microscopic colitis was first coined by Park et al in 1980 as an entity causing persistent watery diarrhea.1 It was initially characterized by chronic watery diarrhea and normal endoscopic findings with abnormal histopathology on biopsy.2 Microscopic colitis comprises two subtypes with lymphocytic colitis and collagenous colitis, based on differing microscopic findings. Lymphocytic colitis is characteristically associated with more than 20 intraepithelial lymphocytes per 100 surface epithelial cells.3 Collagenous colitis is characterized by a thick subepithelial collagen band (more than 10μm) in the basal membrane.3 As awareness and understanding of MC has improved over time, it's documented incidence and prevalence has increased. Although MC was initially conceived to be a histopathologic diagnosis, recent studies have shown specific endoscopic findings that can be associated with MC.4,5 We report two cases of biopsy proven collagenous colitis that had macroscopic features on endoscopic examination.
A 67 year old caucasian female with past medical history significant for chronic obstructive pulmonary disease (COPD), gastroesophageal reflux disease (GERD) and dyslipidemia was evaluated for persistent watery diarrhea without blood or mucus, for two months. She reported concomitant lower abdominal cramps and tenesmus. She denied any weight loss or fever. She endorsed mild improvement in the frequency of diarrhea after the use of Imodium. Her medication regimen at home included Lansoprazole, Escitalopram, Losartan and Fluticasone/Salmeterol. Physical examination did not show any features suggestive of malabsorption or systemic illness. Stool cultures were negative for common bacterial and viral infection. Colonoscopic evaluation revealed a deep solitary rectal ulcer, located 30 cm from the anal verge. Random colonic biopsies were also undertaken from areas of macroscopically normal colonic mucosa. Histopathology from the ulcer showed acute neutrophilic exudate on the background of increased collagen deposition beneath the surface epithelium. Pathology from normal appearing colonic mucosa also showed thickened layer of collagen below the lamina propria consistent with collagenous colitis. Based on these findings, the patient was started on oral budesonide, and Lansoprazole was discontinued. This led to a substantial improvement in symptoms and resolution of diarrhea. Follow up colonoscopy after two months did not show any evidence of mucosal ulceration. Biopsy of the colon revealed improvement in patchy thickening of subepithelial collagen.
A 62 year old caucasian female with a known history of major depressive disorder presented with a two month history of diarrhea. She reported the stool as watery, without any evidence of mucus or blood. She also associated urgency, tenesmus and lower abdominal discomfort with up to fifteen episodes of diarrhea per day. She also reported intermittent epigastric pain and nausea for a similar duration. The patient denied any weight changes, fever, recent travel or use of antibiotics. Her only medication was Venlafaxine. She reported mild epigastric tenderness on examination. Complete blood count and comprehensive metabolic panel did not show any abnormalities. Fecal testing was negative for Clostridium difficile and other infectious agents. In light of epigastric pain, and lower abdominal cramps and diarrhea, esophagogastroduodenoscopy (EGD) and colonoscopy were undertaken. EGD revealed antral gastritis with biopsy findings showing collagenous thickening of the mucosal basement membrane, consistent with collagenous gastritis (Figure 1). Colonoscopic examination was significant for a rectal ulcer (Figure 2), but was otherwise unremarkable for any evidence of colitis. Biopsy from the ulcer and the normal appearing colon was consistent with collagenous colitis (Figure 3). Oral budesonide was initiated and venlafaxine was discontinued. This resulted in resolution of diarrhea and abdominal pain. Budesonide was tapered off with no subsequent recurrence of diarrhea. Follow up EGD and colonoscopy showed resolution of gastritis and rectal ulcer (Figure 4).
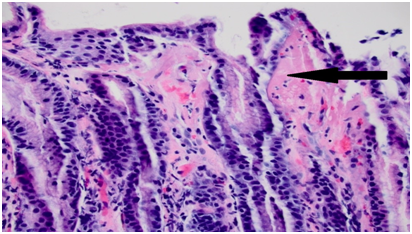
Figure 1 Gastric antral mucosa demonstrating erosive gastritis with overlapping features of collagenous gastritis.
Microscopic colitis is an important differential diagnosis in patients with chronic diarrhea. A systematic review by Tong et al.6 reports an increasing trend in the incidence of MC till the year 2000. The incidence has been stable since then in United States. Overall incidence rate of collagenous colitis (CC) is reported as 4.14 (95% confidence interval (CI) 2.89–5.40) per 100,000 person-years and 4.85 (95% CI, 3.45–6.25) for lymphocytic colitis (LC). Female to male incidence rate ratios were 3.05 (95% CI 2.92–3.19) for CC and 1.92 (95% CI 1.53–2.31) for LC (6). It is a disease of the elderly population. The median age at diagnosis for CC was 64.9 (range, 57.03–72.78) years, similar to LC (median 62.18, range 53.99–70.38).6 Multiple mechanisms of chronic diarrhea in collagenous colitis have been described:
A number of risk factors can predispose patients to develop MC. Several medications have been associated with drug induced microscopic colitis. Proton-pump inhibitors (PPI) appear to have the strongest association.5 In a study done by Beaugerie and Pardi, 10 medications including PPIs, Non steroidal anti inflammatory drugs (NSAIDs), and selective serotonin receptor inhibitors (SSRIs) were ranked as high-risk drugs for developing MC.8 Smoking appears to be an independent risk factor for development and persistence of MC.9,10 Another important consideration is the increased incidence of MC in patients with other autoimmune diseases.11 Associations exist mostly with celiac disease, type 1 diabetes mellitus, autoimmune thyroiditis, or Takayasu’s arteritis may be associated with MC.12 Despite being termed “microscopic” colitis, there have been a number of cases reporting distinct endoscopic findings in patients with collagenous colitis.
Richieri et al.13 first described the presence of multiple linear mucosal lacerations with sharp edges in the right colon of a 43-year-old female, with subepithelial collagen table thickness of 30-40μm, in 1993.13 Since then, there have been more than sixty reports of distinct colonoscopic findings observed in patients with collagenous colitis. Distinct findings include:
Prevalence of mucosal tears as seen in our patients, are estimated to be around 1% based on prior reported studies.4 However, the true number may be higher since not all of these cases are not reported. In addition, practices vary worldwide and up until recently flexible sigmoidoscopy was considered sufficient to diagnose MC. As lesion awareness rises, the incidence of macroscopic findings will increase.
The underlying pathophysiology of mucosal tears has been attributed to thickened abnormal subepithelial collagen that leads to loss of attachment with the epithelial component. This in turn results in stretching of the mucosa over the deeper wall layers, with eventual tearing of the mucosal surface. This sharply demarcated margin of these mucosal defects can help to differentiate these lesions from ischemic colitis.14 Most mucosal abnormalities in collagenous colitis are more likely to be found in the right colon since it has a thinner wall and its expansion to a greater diameter during fecal storage and transit, produces greater relative wall tension.15,16 These abnormalities can be a risk factor of perforation.17 A review by Hussain et al., reports 21 cases of perforation in CC. The majority of these were either colonoscopy associated (15 cases) or barium enema-associated (four cases), while the rest seem to have occurred spontaneously.18
In a study of 795 patients, Mellander et al.,19 found endoscopic abnormalities in 37% of patients with CC and 25 % of patients with LC. However, this spectrum of endoscopic abnormalities is seen in other diseases of the colon as well, thus making these findings non specific. Microscopic colitis is diagnosed on the basis of characteristic histologic findings, adequate endoscopic sampling is key. It is recommended to take at least two biopsies per segment, to ensure a high degree of likelihood of detect findings suggestive of CC or LC.20 Key diagnostic components for the diagnosis of MC is defined as subepithelial collagenous band >10μm in thickness for CC and increase of intraepithelial lymphocytes (>20 per 100 epithelial cells) for LC.21
Once MC is diagnosed, the first step in treatment is to identify risk factors such as inciting medications or smoking. In mild cases, discontinuation of medications and use of antidiarrheal medications can lead to resolution.5 Data from randomized trials indicates that Budesonide (9mg up to 8 weeks) increased the probability of experiencing clinical remission by 152% after 6 to 8 months of follow-up (RR, 2.52; 95% CI, 1.45-4.4). Clinical response was also matched by histologic remission in these studies.22,23 The mean time to induce remission in patients with collagenous colitis was found to be 7 to 13 days.23 The overall response may be expected for up to 81% of MC patients. Regarding maintenance of histological response, meta-analysis showed that budesonide 6 mg reduces the risk of histological relapse by 79% (RR, 0.21; 95% CI, 0.08–0.54).24 Other medications that have shown some benefit include bismuth salicylate or mesalazine plus cholestyramine, but they have lesser possibility of successful remission, compared to budesonide.25 In severe or refractory patients, immunomodulatory medications (TNF alpha inhibitors) have been tried,26 however no large scale studies have shown consistent benefit. Surgery can be considered in patients with severe disease, however this remains a treatment of last resort.
Our unique cases describe macroscopic features in patients with collagenous colitis as well as the correlation with SSRI. Currently there is no data available that links specific endoscopic findings with medication induced microscopic colitis. Our report would help serve as a starting point for further studies that can help answer this question.
None.
The authors declare no conflicts of interest.
None.

©2017 Asad-ur-Rahman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.