eISSN: 2373-6372


Research Article Volume 7 Issue 1
1Department of Transplant Pharmacy, The Ohio State University-Wexner Medical Center, USA
2Department of Surgery, The Ohio State University-Wexner Medical Center, USA
3Center for Biostatistics, The Ohio State University-Wexner Medical Center, USA
4Department of Medicine, The Ohio State University-Wexner Medical Center, USA
Correspondence: Khalid Mumtaz, Assistant Professor, Division of Gastroenterology, Hepatology & Nutrition, Wexner Medical Center, The Ohio State University, Columbus, OH, 43210, USA, Tel 614-685-8657
Received: June 13, 2017 | Published: June 28, 2017
Citation: Pastrana-Camacho T, Bradley M, Winters H, Black S, Porter K et al. (2017) Multidisciplinary Approach to treat Liver Transplant Recipients with Hepatitis C using Sofosbuvir based Therapy. Gastroenterol Hepatol Open Access 7(1): 00227. DOI: 10.15406/ghoa.2017.07.00227
Introduction: The safety and efficacy of the direct acting antivirals in liver transplant recipients is unknown.
Materials and Methods: Retrospective cohort study on 44 liver transplant recipients, ≥18years old who received Sofosbuvir (SOF) based antiviral therapy (AVT) at our hospital from January 2012 through May 2016. Multidisciplinary (MDT) approach involving hepatologists, transplant pharmacists and a patient access coordinator was adapted. SVR at 12 and 24weeks, safety and compliance of SOF based AVT were reported. We also reported on improvement in APRI and CPT score at SVR.
Results: 35 patients (79.5%) were treated with Ledipasvir (LDV) /SOF, 7 (16 %) with SOF/RBV and 2 (4.5%) with SOF/Simeprevir (SIM). Most patients were HCV genotype 1 (n=37; 84.1%) and treatment experienced (n= 24; 55%). Median time between liver transplant (LT) and AVT was 2.7years with majority (66%) treated ≥12 months after LT. Median pre-treatment HCV viremia was 3,800,000 (71,000-66,000,000) with a high viral load ≥800,000 copies/ml in 36 (82%) patients. SVR 12 and 24 were achieved in 43/44 (98%), despite a low (77.3%) rapid virological response. Statistically significant improvement was observed in pre-treatment and post- SVR median albumin levels, APRI and CPT scores. Fatigue (27%) was the most commonly reported adverse effect. Compliance rate was 100%.
Conclusion: With a MDT approach, using SOF based AVT, a very high SVR was achieved in liver transplant recipients with recurrent HCV infection. A MDT approach positively impacts medication acquisition, enhances patient education and compliance in post LT HCV management.
Keywords: multidisciplinary, HCV, liver transplantation, antiviral therapy, direct antiviral agents, sofosbuvir
SOF, sofosbuvir; AVT, antiviral therapy; MDT, multidisciplinary team; LDV, ledipasvir; SIM, simeprevir; HCV, hepatitis c virus; LT, liver transplantation; IS, immunosuppressive; SVR, sustained virological response; GT, genotype; DAA, direct acting antiviral; CPT, child-pugh-turcotte; TP, transplant pharmacist; PAC, patient access coordinator; AASLD, american association for the study of liver diseases, RBV, ribavirin, APRI, ast to platelet ratio index; LTRs, liver transplant recipients
Hepatitis C virus (HCV) presents a major health care challenge and is the leading indication for liver transplantation (LT) in the United States.1,2 Recurrence of HCV after LT is universal and contributes immensely to graft failure and early mortality due to immunosuppressive (IS) therapy related acceleration of fibrosis.3 Treatment with AVT and eradication of virus is the only way to improve the outcomes related to recurrence of HCV.
Until 2011 pegylated interferon plus ribavirin was the standard of care for HCV treatment, resulting in a sustained virological response (SVR) in around 30%-50% of transplant recipients with HCV genotype 1(GT).4–11 A major change in paradigm has been observed with the discovery of safe and effective direct acting antiviral (DAA) therapy in the field of HCV treatment.12 These DAAs have already been shown to change the landscape of AVT for HCV infection in non-transplant and post LT settings. Recently published randomized controlled studies on the use of sofosbuvir based AVT reported SVR rates ranging between 70% to 85% in post LT patients with child-pugh-turcotte (CTP) A; B and C patients.4,13,14 Overall; the treatment was well tolerated; with few severe side effects. Similar results were reported in studies based on real world data such as TRIO and TARGET 2.0 trials.15–18 A recent real world data-based study published from Canada on efficacy of SOF-based treatment reported overall SVR rates of 85% in all genotypes.19 However; these results are not as encouraging as reported in non-transplant setting with SVR of ~95%.20–22 Besides HCV genotype; and stage of fibrosis; compliance to therapy is a very important predictive factor of SVR.23 Post LT patients are on multiple medications besides their immunosuppression including prophylaxis for bacterial and viral infections; bisphosphonate, antihypertensive, anti-diabetic medications, etc. They also can suffer from memory impairment due to their post- transplant course, previous history of drinking and IS use. Moreover, substance abuse and psychiatric illnesses are also common in hepatitis C patients.24 All these factors may lead to issues with adherence to very expensive DAA for recurrent HCV infection resulting into lesser efficacy. Multidisciplinary team approach has been studied and reported to be successful in the era of interferon based AVT for patients with HCV infection in a non-transplant setting.25 Improvement in patient compliance and the efficiency of antiviral treatment has already been reported in the non-transplant setting.
Recent advancements in discovery of various DAA may allow more patients to have access to life altering medications. However, many hurdles exist including high cost of treatment; drug-drug interaction and availability of long-term data in transplantation.26–28 Once the medication is approved; there exists a litany of drug interactions in the transplant population.28 Moreover; logistic and compliance issues alluded above may also contribute to outcomes of the DAA based treatment in recurrent HCV patients. In order to tailor a SOF based AVT regimen in patients with recurrent HCV infection; we employed a MDT approach from the initial encounter, approval of medication to the monitoring of drug safety; interaction, compliance and ultimately completion of AVT. Our MDT approach included involvement of Hepatologists, Transplant Pharmacists (TP) and Patient Access Coordinator (PAC) in care of post LT HCV patients.
Study population and design
This is a retrospective; single center cohort study approved by the Institutional Review Board of our institution. All adult (age ≥18) liver transplant recipients (LTRs) (treatment experienced and naïve) with recurrent HCV infection treated with SOF-based AVT between January 2012 to May 2016 were included. Recurrent HCV infection was diagnosed based on HCV viremia in the post LT setting. Based on the American Association for the Study of Liver Diseases (AASLD) guidelines, a liver biopsy is not a prerequisite to qualify for treatment.
The patients in the current study were treated with SOF based AVT with or without Ribavirin (RBV) for 12 or 24weeks. Regimens of SOF based AVT included LDV/SOF (n=35); SOF/RBV (n=7) and SOF/SIM, (n=2). Ribavirin dose was adjusted according to weight of patient and their renal function. Starting dose of RBV was 600 mg daily and increased to 1000 mg if patient weighs <75kg or 1200mg in patients weighing ≥75kg. This dose could be reduced based on the tolerance and level of hemoglobin. No changes were made to the ongoing IS regimen. Patients with chronic kidney disease (GFR<30mL/min), HIV/HCV co-infection, HBV/HCV co-infection, prisoners and pregnant females were excluded.
Multi-disciplinary approach
Patients were assessed by a Hepatologist in the transplant clinic for SOF based AVT. Based on the eligibility criteria, patients underwent required laboratory testing in order to prepare for the prior authorization required for AVT. Patients were assigned treatment based on the AASLD/ Infectious diseases society of America (IDSA) guidelines and practitioner expertise in a non-randomized process. All patients eligible for SOF based DAA were referred to a TP and PAC. The purpose of referral to pharmacist was to further emphasize on the compliance of AVT, reviewing insurance approval procedure, identifying drug-drug interactions, and educate the patient on possible side effects of treatment. The PAC ensured that appropriate labs were ordered after explaining their necessity to patients and obtained their consent to draw. After appropriate labs were obtained; the prescription for the medication(s) was sent to The Ohio State University Specialty Pharmacy in order to initiate the prior authorization process. At this point, a specialized prior authorization technician filled out the proper forms with resulted laboratory values and reason for HCV treatment. If initially denied; the technician collaborated with the Hepatologist and TP to compose a letter outlining the need for HCV treatment. Throughout this time period, the PAC kept communication and support open to the patient while they waited for approval. After treatment approval, the TP at the Specialty Pharmacy would contact the patient to reinforce education provided during initial assessment visit. On each follow up clinic visit, TP monitored for response to treatment, possible drug interactions, need for medication assistance program referral and/or therapy modification.
Efficacy and safety assessment
Blood work including complete blood counts; creatinine; electrolytes; liver function tests and INR were monitored at baseline and then at every 4week after the start of SOF based AVT. Complete blood count was monitored every other week in patients on RBV and the dose was adjusted according to hemoglobin levels. HCV RNA PCR was checked using ELISA immunoassay at week4 week 12 or end of treatment (limit of detection, up to 12IU/ml). SVR was considered as an undetectable HCV viral load 12 and 24weeks after the end of treatment. Levels of calcineurin inhibitor were also monitored during and after the treatment as per our transplant center protocol.
Study outcomes
Sustained virological response at weeks 12 and 24 in LTRs with recurrent hepatitis C was reported. Secondary outcomes included side effects, drug-drug interactions and compliance. We also reported improvement in AST to platelets ratio index (APRI) score, responses at 4weeks & at the end of treatment, and improvement in post AVT albumin levels.
Statistical analysis
Patient characteristics are presented as median and range for continuous variables and number and percent for categorical variables. The percentage of patients achieving SVR-12 and -24 is reported. Pre- and post -AVT variables including AST to platelets ratio index score; and albumin were compared using Wilcoxon sign rank tests. Pre-AVT and post SVR CTP score were also compared using McNemar’s tests for paired data.
Baseline characteristics
A total of 133 LTs were performed at our institute during the study period. Of these 59 (44%) underwent LT for HCV cirrhosis related complications, 44 with recurrent HCV infection were treated with SOF-based AVT. Of 37 GT-1 patients 35 (79.5%) were treated with SOF/LDV and two (4.5%) received SOF/SIM. Seven (16%) with GT-3 treated with SOF/RBV and. The majority of patients were placed on 24 weeks of therapy; however due to insurance constraints 2 patients in the SOF/LDV group and 1 patient in the SOF/SIM group were treated for 12 weeks with the addition of RBV. Baseline characteristics of the patient population are summarized in Table 1. Majority (79.5%) were males with a median age of 61 years (range; 41-70 years). Median HCV viral level was 3,800,000 (71,000-66,00,0000) copies /ml and 36 (82%) had a high baseline viral load of³ 800,000 copies/ml. Thirty-seven (84%) were genotype 1and of these 29 (78%) had GT-1A infection. Severity of liver disease was assessed with the help of APRI and CTP score. Median APRI score of our cohort was 0.78 (range: 0.21-7.36). There were 40 (91%) and 4 (9%) patients with CTP-A and CTP-B respectively. None of the patients had decompensated liver disease at initiation of AVT. There were 24 (54.6) treatment experienced and 20 (45.4%) treatment naïve patients in our cohort. Median time between LT and start of AVT was 2.7years and 34 % got treatment within 12months from LT.
Characteristic |
Median (Range) or n (%); n=44 |
Age (years) |
61 (41-70) |
Gender |
|
Female/Male |
9 (20.5)/ 35 (79.5) |
Race |
|
African American |
8 (18.2) |
White |
36 (81.8) |
BMI |
29 (17-67) |
Diagnosis |
|
HCV only |
14 (31.8) |
HCV/ETOH |
6 (13.6) |
HCV/HCC |
24 (54.6) |
Genotype- 1 |
37 (84) |
1A & 1B |
29 (78.4) & 8 (21.6) |
Genotype Non- 1 |
7 (16) |
Immunosuppression |
|
Tacrolimus |
21 (47.7) |
Cyclosporine |
23 (52.3) |
Mycophenolate Mofetil |
40 (91%) |
Previous Treatment |
|
Treatment naïve |
20 (45.4) |
Treatment experienced |
24 (54.6) |
SOF-based treatment |
|
SOF/LDP |
35 (79.5) |
SOF/RBV |
7 (15.9) |
SOF/SIM |
2 (4.5) |
Cirrhosis |
3 (6.8) |
HCV PCR > 800K copies/ml |
36 (81.8) |
Inflammation grade >1 |
11 (25) |
Fibrosis stage ≥ F1 |
16 (36.4) |
Median time between LT and AVT (yrs) |
2.7 (0.5 – 17) |
Time from LT to AVT <12months |
15 (34.1) |
Table 1 Baseline characteristics of patients
Treatment response
Overall 43 (97.7%) patients out of 44 treated achieved SVR-12 and -24. Of these 32 (72.7%) achieved undetectable HCV RNA at week-4 and 43 (97.7%) had end of treatment response. No patients experienced virological breakthrough while on SOF-based regimens or relapsed. The only patient who did not respond to a 24 weeks of SOF/RBV combination had HCV GT-3 infection and was treatment naive. There were no differences in SVR rates for subgroups based on age (<60 vs ≥60years); gender (male vs female) ethnicity (White vs African American) prior treatment status (treatment naïve vs treatment experienced) and HCV viremia (<800K vs ≥800K) levels (Figure 1). There was statistically significant improvement in median APRI score (0.78, range: 0.21-7.36 to 0.32, range: 0.12-1.15, p<0.001) median albumin (3.9grams/L, range: 2.6-4.8 to 4.0grams/L, range: 3.0-5.0, p=0.01), and CTP score
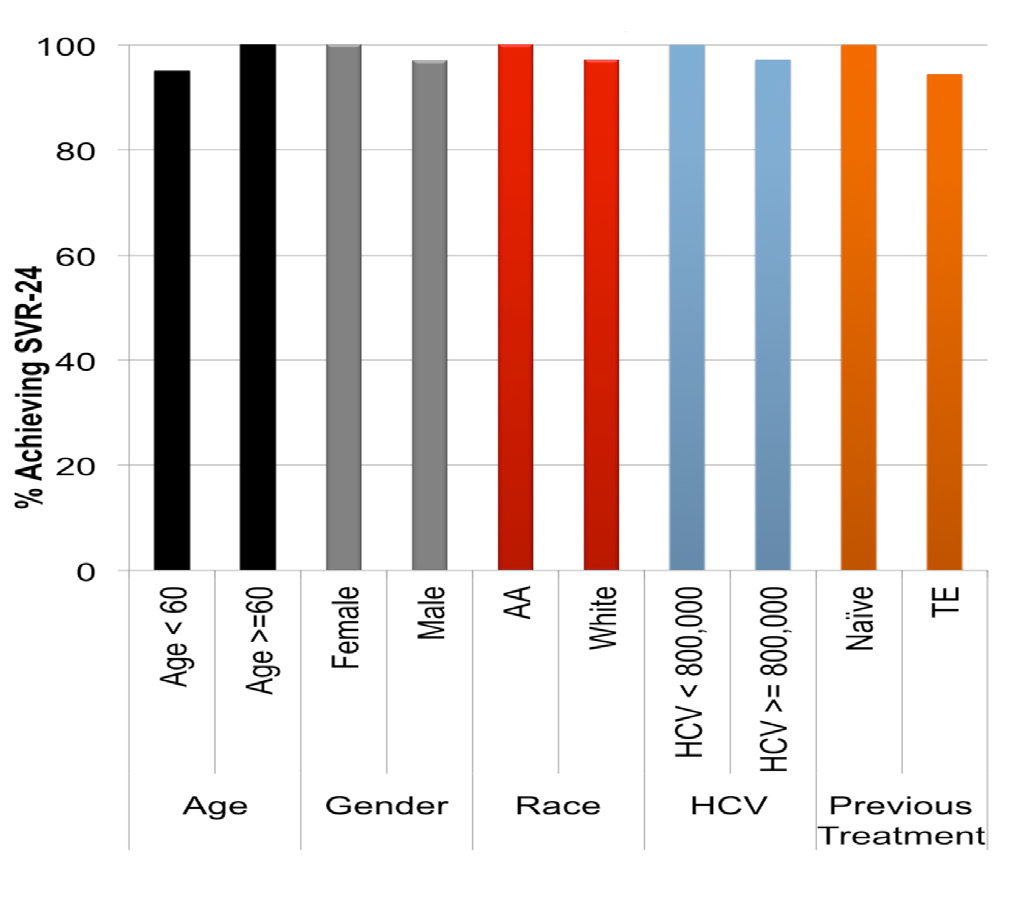
Figure 1 Sustained Virological response is not dependant on age; gender; race; HCV viremia and prior treatment response.
*Sign rank tests for paired differences
**Median INR was equal but distribution was higher for Pre SOF.
Safety
Treatment was very well tolerated with majority n=28 (63.6%) reporting no side effects (Table 3). Sixteen patients (36%) reported adverse events; all of which were mild and did not require treatment discontinuation. In those reporting adverse events; the most common were fatigue (27%) headache (9%) and insomnia (9%). None of the patients had graft rejection or died and all were able to complete the treatment.
|
Pre SOF (n=43) |
24 weeks Post SOF (n=43) |
p-value* |
Hemoglobin (gm/L) |
12.8 (8.5-15.4) |
12 (8.6-16.9) |
0.94 |
INR** |
1.0 (0.9-1.6) |
1.0 (0.9-1.2) |
<0.001 |
Creatinine (mg/dl) |
1.31 (0.62-2.60) |
1.22 (0.70-2.40) |
0.13 |
ALT (IU) |
46 (11-322) |
17 (6-51) |
<0.001 |
AST (IU) |
48 (14-377) |
20 (12-46) |
<0.001 |
Alkaline Phosphate (IU) |
89 (8-308) |
80 (45-190) |
0.01 |
Bilirubin (mg/dl) |
0.8 (0.3-2.8) |
0.7 (0.1-1.8) |
0.11 |
Albumin (g/L) |
3.9 (2.6-4.8) |
4.1 (3.0-5.0) |
0.001 |
CTP Score |
5 (5-7) |
5 (5-6) |
0.02 |
APRI Score |
0.78 (0.21-7.36) |
0.32 (0.12-1.15) |
<0.001 |
|
|
|
|
HCV PCR |
3;800;000 (71;000-66;000;000) |
6 (0-286) |
<0.001 |
Table 2 Changes in pre AVT and post SOF-based treatment parameters
Side Effects |
n=44 (%) |
Fatigue |
12 (27.3) |
Headache |
4 (9.1) |
Nausea |
3 (6.8) |
Insomnia |
4 (9.1) |
Anemia |
3 (6.8) |
Decompensation |
0 |
Treatment Discontinuation |
0 |
No Adverse Effects |
28 (63.6) |
Table 3 Adverse events on SOF based anti-viral therapy
Compliance
With the help of our multidisciplinary team approach all patients were able to complete the SOF based AVT. None dropped out reported missing a single dose of the medication or was lost to follow-up. Therefore our study medication compliance was 100%.
In this study we describe our experience of treating recurrent HCV infection in LTRs including mainly genotypes 1 and 3 with SOF-based regimens adapting a multi-disciplinary approach. Very high SVR rates were achieved with the help of SOF-based AVT and treatment was very well tolerated with 100% compliance rate. During treatment; there were no antiviral therapy related serious adverse effects or significant drug-drug interactions noted. Only one patient was unable to achieve SVR belonging to HCV genotype 3 which has emerged as the most challenging of all HCV genotypes to treat despite the introduction of newer direct-acting antiviral therapies.
Previous studies have shown that overall graft and patient survival are lower in liver transplant recipients with HCV infection than among recipients without HCV infection due to its universal recurrence and rapid progression to cirrhosis. In the present study; the SVR rates were comparable among all treatment regimens and the addition of RBV had no impact on treatment or SVR rates. SVR at 24weeks in this entire cohort was 98%. This is comparable to preliminary results of a phase 2 study presented at the 2014 AASLD meeting using a fixed dose combination of LDV/SOF with RBV for 12 or 24weeks post-LT patients with genotype 1 or 4 with recurrent HCV post transplantation.14 Our results are consistent with a recently published trial which evaluated combination of ritonavir with paritaprevir ombitasvir; plus dasabuvir and RBV for 24weeks in 34 LT recipients with GT-1 infection with METAVIR ≤ F2 fibrosis.29 Authors of this trial demonstrated high efficacy with an overall SVR-12 rate of 97% which is comparable with our experience in patients with milder recurrent HCV infection. A recent study in which 12 patients with recurrent HCV after liver transplantation received sofosbuvir with and without RBV concluded that optimal outcomes require initiation of treatment before decompensation.30 Based on that we recommend initiating AVT in mild disease to get maximum outcome in LT recipients.
Other reports have found variable responses with different HCV genotypes. Faisal et al and Pungpapong et al reported lower response in GT-1A LT recipients at 76%.19,31 Gutierrez et al.,32 have also reported similar results in their respective cohorts of GT-1A patients.32 Patients with HCV GT-2,3 and 4 were studied by Faisal et al and reported SVR rates were 83%, 100%, and 75%, respectively.19 Another study reported SVR of 93% with 12weeks of treatment in GT-2 in the non-transplant population.33 In our study despite fewer patients SVR was 100% and 86% among GT-1 and GT-3 respectively. The only patient who didn’t respond to AVT had HCV GT-3 infection. Now it has been established that genotype 3 has inferior results in new era of oral SOF based AVT.34,35 Only 16 patients in our study underwent liver biopsy. Therefore we assessed the severity of recurrent HCV infection with the help of albumin; APRI and CTP score. We found an improvement in albumin APRI score and CTP score in our cohort after successful completion of AVT. Study by Pellicelli et al also showed improvement in CTP and MELD scores after completion of therapy; which is consistent with our findings.30
Furthermore most factors previously associated with inferior response such as age gender ethnicity viral load and previous treatment history don’t hold the same importance with SOF-based regiments as they did for interferon-based therapy. Our findings suggest that most baseline characteristics and lack of viral response during AVT may no longer be relevant predictive factors when treating patients with SOF-based regimens. A very recent study by Welzel et al.,36 also showed that on-treatment HCV RNA quantification is of limited clinical use in patients with advanced liver disease and/or liver transplantation and does not predict SVR12.36 Faisal et al.,19 have reported similar results in their observational study which evaluated the efficacy safety and tolerability of regimens containing sofosbuvir in the treatment of HCV recurrence in all genotypes outside of clinical trials in all Canadian transplant centers.19
In our study doses of calcineurin inhibitors were monitored during the treatment period to maintain therapeutic levels and no dose adjustment was required. No deaths or episodes of graft rejection occurred in this population. The most common side effects were fatigue; headache and insomnia; seen in 27%, 9% and 9%, respectively, no patients discontinued treatment due to adverse events. There was no need for blood products for patients on RBV.
To our knowledge this is the first study reporting on role of multidisciplinary team approach including hepatologists specialty pharmacist and a patient access coordinator in LTRs using DAA for recurrent HCV infection. An earlier study in Interferon based AVT era also assessed the importance of MDT comprising two hepatologists two nurses one pharmacist one psychologist one administrative assistant; and one psychiatrist. They reported increased efficiency and compliance in MDT approach as compared to historic control group.25 The management of HCV infections is centered on pharmacotherapy making the clinical pharmacist appropriately suited as part of the patient’s care team. Direct acting antiviral medications used to treat HCV-infected patients are effective in achieving SVR. However the monitoring of adverse effects significant drug interactions and high risk of non-adherence and treatment discontinuation demands a transplant clinical pharmacist involvement in managing this expensive treatment.37 Accumulating evidence suggests that a pharmacist as part of a multidisciplinary team can have beneficial effects on patient care. Pharmacist-provider team-based care is widely supported in the literature demonstrating significant improvement in cardiovascular and renal outcomes.38–40 Our findings suggest that role of a pharmacist based education and a patient access coordinator in facilitation on medication acquisition and improved patient outcomes which reflects the importance of adopting a multidisciplinary approach in HCV management. We found that median time between LT and AVT therapy start was 2.7years and 34% were able to get the treatment in less than 12months after LT. Early acquisition of DAA was achieved with the help of our access coordinator which ultimately resulted in improved treatment efficacy and compliance.
There are a few limitations that should be considered in the interpretation of this report including its small sample size which is insufficient to allow subgroup comparisons and characterization of efficacy in certain populations (i.e. African Americans HCV genotype 3). In addition our population consisted of patients with well-compensated liver disease so we could not study the efficacy of DAA in patients with advanced disease. Lastly we did not have a comparison cohort to compare the MDT approach and assess its role.
In conclusion a multidisciplinary team approach was found to be helpful in improving patient compliance and increases the efficiency of SOF-based antiviral therapy. We found that a close cooperation among the healthcare providers in the care of post LT patients with recurrent HCV infection can ensure optimal treatment performance and high SVR rates.
Authors would like to thank Dr. Anthony J. Michaels who provided useful suggestions in final version of our manuscript.
The authors declare that there is no conflict of interest regarding the publication of this paper.
The authors have no funding to report.
None.

©2017 Pastrana-Camacho, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.