eISSN: 2373-6372


Research Article Volume 6 Issue 4
1Department of Gastroenterology, Dr. Sulaiman al Habib Medical Group Olaya Branch Obesity Unit, Saudi Arabia
2Investigation Unit, Costa del Sol Health Agency, Spain
Correspondence: Cristobal Albandea Moreno, Department of Investigation Unit Pasaje Miramar del palo 18,6-D.29017 Malaga, Spain, Tel 27.86489363
Received: March 04, 2017 | Published: March 30, 2017
Citation: Moreno CA, Ibrahim BS, Rivas-Ruiz F, Habib S (2017) Swallowable Obalon Gastric Balloon for Obesity: Pilot Study. Gastroenterol Hepatol Open Access 6(4): 00206. DOI: 10.15406/ghoa.2017.06.00206
The goal of this study was to evaluate the safety and the impact on weight loss of a new swallowable gastric balloon. In this prospective pilot study, 87 overweight or obese patients were included. Up to two balloons were ingested under fluoroscopic control. All balloons were removed by upper GI endoscopy, 12weeks after the ingestion of the first balloon. 74 out of 87 attempts (85 %) to swallow a balloon were successful. Nausea and stomach pain were the most frequent side effects. Endoscopic procedures for balloon removal were uneventful. Weight loss was significant at week12. This pilot study showed no significant side effects induced by up to two balloons, and a significant weight loss.
Keywords: gastric balloon, obalon, weight loss
OGB, obalon® gastric balloon; BMI, body mass index; UBT, urea breath test; AEs , adverse e; GI, gastrointestinal; PPI, proton pump inhibitor
Background
Intragastric balloons, filled with liquid or air, have been used for years to induce weight loss in obese patients,1,2 which results may be superior to diet alone.3,4 They require the use of upper gastrointestinal (GI) endoscopy to place the balloon in the stomach and remove it at the end of the treatment period. These endoscopic procedures increase medical risks and costs of intragastric balloons.5 The ability to adjust balloon volume in the gastric cavity has also been shown to improve tolerance and efficacy in order to induce a significant weight loss.6 A new gas filled, swallowable intragastric balloon, has been designed to minimize the need for upper GI endoscopy to removal only and allowing gastric volume titration by swallowing additional balloons, has been developed to improve the limitation of currently available devices.We reported here the results of a pilot study to evaluate the safety and efficacy in terms of weight loss over a 12-week period of this device.7
Objective
The aim of the pilot study is to evaluate the safeness and efficacy of swallowable Obalon Intragastric Balloon on adult population
The Obalon® Gastric Balloon (OGB, Obalon TherapeuticsInc, Carlsbad, CA, USA) is a gas-filled balloon with a maximal volume of 250ml. The balloon contains a self-sealing valve connected to a thin catheter. Once the capsule is ingested, the catheter extends from the stomach to outside the body through the esophagus and the mouth. Fluoroscopy is used to verify that the capsule has entered the stomach. The catheter is attached to the balloon for remote inflation using a gas-filled canister. After balloon inflation, the catheter is detached and removed. Balloon removal is performed by upper GI endoscopy, under sedation without tracheal intubation.
We enrolled 74 adult patients with overweight and obesity. In all patients anthropometric parameters were evaluated at the time of insertion and after withdrawal of balloons. We also evaluated capacity of the patients for swallowing the Obalon intragastric balloon and the side effects after insertion. Patients willing to participate with a BMI between 26 and 47kg/m2 (patients with BMI>40 refused to do bariatric surgery), signed the informed consent form prior to any procedure. H. pylori testing was performed in all cases by serology: H. pylori-positive patients were either treated (and H. pylori eradication was confirmed by a urea breath test (UBT) prior to further procedures), or excluded from the study. Patients were requested to take a standard dose of PPI once per day for the 12weeks after ingestion of the first balloon to 7days after balloons removal and to avoid gastric toxic drugs such as aspirin or NSAIDs during the entire duration of the study. After the ingestion of the first balloon, patients were provided basic nutritional counseling. The patients were also provide with medical treatment to control the device-related symptoms such as stomach pain, cramping, nausea or vomiting. Patients were seen every other week to monitor tolerance and adherence the nutritional recommendations. The second balloon were inserted after 1week from the first one. All balloons were then endoscopically removed 12weeks after the ingestion of the first balloon.
A first and second balloon was ingested successfully by 74 subjects. The mean time for ingestion procedures (swallowing of the capsule and inflation of the balloon) was 9min (range: 5-22min). All balloons were removed by upper GI endoscopy, under conscious sedation without tracheal intubation. At the time of withdrawal, we found 2 subjects with 1 balloon deflated (these 2 subjects came for withdrawal after 4months, and the balloons were expelled through the stools). 10 gastric superficial erosion lesions were observed in 10 subjects, probably in relation with using NSAIDs and without proton pump inhibitor (PPI). The balloons were removed without any difficulty in all cases. Stomach pain/cramping was the most common adverse event occurring in 65 (87%) subjects, being treated with oral antispasmodic medical therapy, and 18 (24%) subjects reported Nausea, treated by oral domperidone. All reported adverse events (AEs) were mild to moderate at most. The number of AEs was similar with one and two balloons. No reported cases of removing balloons before 12weeks. This pilot study showed significant decrease of weight and body mass index at 12weeks, with high rate of successfully swallowing and transient side effects induced by up to two balloons (Figures 1-5).
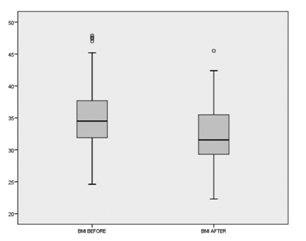
Figure 1 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
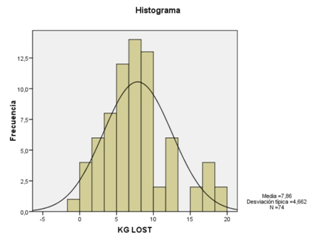
Figure 2 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.

Figure 3 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
Results from this pilot study supports that this swallow able balloon can be administered easily, with high rate of successfully swallowing, without complications, getting an excellent tolerance and inducing within a 3-month a significant weight loss and reduction Body Mass Index (BMI). Two of the advantages of this swallow able intragastric balloon are, on the one hand, it limits the number of the endoscopy procedures under conscious sedation, minimizing the risk of tracheal aspiration during the endoscopy procedure, and on the other hand, it allows for a progressive filling of the gastric cavity, which represents potentially enhancing tolerance, and in consequence, efficacy of the treatment. The endoscopic removal under conscious sedation was possible in all cases. Regarding the issue of the deflated of the balloons, it is a rare case; in our study happened only in 2 patients, clearly in relation with the delayed time for withdrawal, after the established period of 3months.
None.
Author declares there are no conflicts of interest.
None.

©2017 Moreno, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.