eISSN: 2373-6372


Case Report Volume 6 Issue 1
1Fellow in Gastroenterology of the Hospital Edgardo Rebagliati Martins, Peru
2Head of the Therapeutic Endoscopy Service of the Hospital Edgardo Rebagliati Martins, Peru
3Assistant in Gastroenterology of the Hospital Edgardo Rebagliati Martins, Peru
Correspondence: Augusto Vera Calderón, Head of the Therapeutic Endoscopy Service of the Hospital Edgardo Rebagliati Martins, Peru
Received: December 15, 2016 | Published: February 2, 2017
Citation: Sinagra E, Matrone R, Gullo G, Catacchio R, Renda E, et al. (2017) Clinical Efficacy of Ginger Plus B 6 Vitamin in Hyperemesis Gravidarum: Report of Two Cases. Gastroenterol Hepatol Open Access 6(1): 00182. DOI: 10.15406/ghoa.2017.06.00183
Endoscopic drainage of pancreatic and peri pancreatic mature collections guided by USE actually is recognized as an alternative to other therapeutic modalities such as percutaneous and surgical interventions, because it is safe and achieves high success rates, being higher for pseudocysts. In this case series, we describe our initial experience in transmural endoscopic ultrasound guided drainage carried out with biliary plastic double pigtail stents in five patients with this type of collections secondary to acute pancreatitis.
Keywords: pancreatic pseudocyst, encapsulated necrosis, endoscopic ultrasound guided drainage, acute pancreatitis
Pancreatic and peri-pancreatic collections may develop secondary to fluid leakage or liquefaction of pancreatic necrosis following acute pancreatitis, chronic pancreatitis, surgery or abdominal trauma.1 According to the Atlanta classification,2 acute collections (evolution time less than 4 weeks) are the acute peri-pancreatic fluid collection and acute necrotic collection that rarely become infected and usually resolve spontaneously.3 On the other hand, late complications are pseudocysts (a peri-pancreatic or pancreatic circumscribed fluid collection that persists for more than 4 weeks) and encapsulated necrosis or WON (walled off necrosis), which may be of pancreatic or extra pancreatic origin, which appears after 4 weeks of initiation of necrotizing pancreatitis.4
The diagnosis of these lesions were initially made by contrast-enhanced tomography; however, that magnetic resonance cholangiopancreatography(MRCP) is more precise to define the content of these collections (such as the presence of detritus), which allows the differentiation between WON or pseudocysts, verify if there is communication with the main pancreatic duct.5 Evaluation by Endoscopic retrograde cholangiopancreatography (ERCP) can be made prior to definitive therapy to define anatomy and guide the therapy.6
The purpose of the present case series is to describe our initial experience in the endoscopic management of inflammatory pancreatic or peri-pancreatic collections (mainly pseudocysts) secondary to acute pancreatitis, through endoscopic drainage guided by USE performed by an expert endoscopist in the Hospital Nacional Edgardo Rebagliati Martins.
Case 1
A 60-year-old male patient with a past medical history of severe acute pancreatitis (probable biliary etiology) with ICU management, hypertension and obesity. He presents 2 years later complaining of abdominal pain and bloating. Abdominal palpation revealed pain in the epigastrium. Laboratory results: normal. MRCP: an 18 x 6 cm pseudocyst with detritus in the pancreatic body and tail without communication with the main pancreatic duct. Endoscopic drainage was made using a 19G needle to puncture, placement of two 7F double Pigtail stents. Technical, clinical (asymptomatic at 5 months) and radiological success were obtained. In USE control (3 months later), there were signs of chronic pancreatitis (Figures 1 & 2).
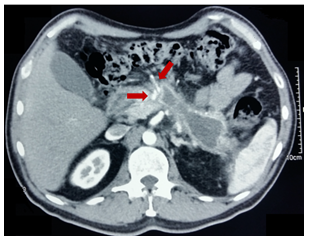
Figure 1 Contrast-enhanced CT: pseudocyst of the body and tail of pancreas with decrease in dimensions 5 months after endoscopic drainage with stents in situ (arrows).
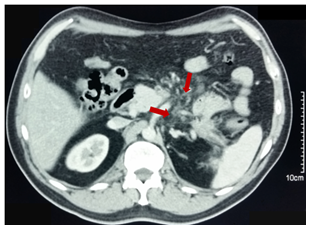
Figure 2 Contrast-enhanced CT: residual inflammatory process (arrows) without evidence of pseudocyst (6 months after drainage).
A 72-year-old female patient with a history of acute biliary pancreatitis, arterial hypertension, type 2 diabetes mellitus, and cholecystectomy. She presents 10 years after pancreatitis with 1 year of abdominal pain. Physical examination: evident protrusion and painful palpation in the left upper abdominal quadrant. Normal laboratory results. Contrast-enhanced CT and MRI showed an 11 x 9 cm pseudocyst. It was punctured with a 19G needle and two 9 cm x 7F double Pigtail stents were placed (Figures 3 & 4). After 6 months of follow-up, only residual inflammation was evident and the stents were subsequently removed. Favorable clinical outcomes (Figures 5 & 6).
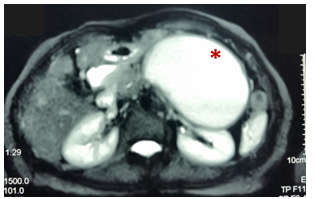
Figure 3 Pre-drainage MRCP (T2 weighted image): Pseudocyst of the body and tail of pancreas (asterisk) that exerts compressive effect on stomach, small bowel and left kidney.
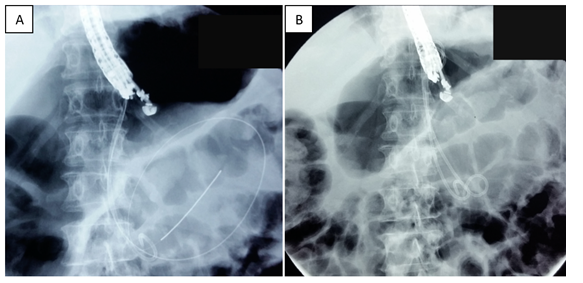
Figure 4 Fluoroscopic images of endoscopic drainage. A. Passage of guidewires. B. Successful placement of two double Pigtail stents.
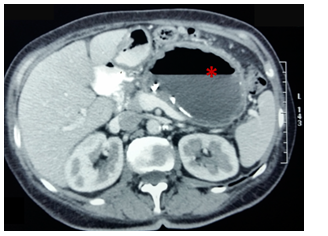
Figure 5 Contrast-enhanced CT: (15 days after drainage). Dimensions decrease of the pseudocyst (asterisk). With hidroaereos level in this one with stent in situ.
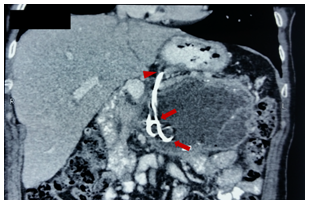
Figure 6 Contrast-enhanced CT: (15 days after drainage). 2 double Pigtail stents in situ between stomach (arrowhead) and the pseudocyst (arrows).
Case 3
A 57-year-old woman with a history of severe acute biliary necrotizing pancreatitis (necrosis of 40% of the gland); type 2 diabetes mellitus and controlled hypothyroidism; cholecystectomy (10 years before pancreatitis) and appendectomy. She was admitted 3 years after acute pancreatitis with a course of 1 year of abdominal pain. Physical examination: painful deep palpation in epigastrium. Laboratory results: ALT: 72 IU/ L; Alkaline phosphatase: 153 IU/ L. MRCP: a 10 cm sized pseudocyst in the body of pancreas that produces dilatation of the main pancreatic duct and mechanical effect on the gastric cavity. It was punctured with a 19G needle on the posterior aspect of the gastric body and 2 double Pigtail stents (one of 9 cm x 7F and another of 7 cm x 7F) were placed. After the procedure the patient suffered a post-drainage infection that resolved with intravenous broad-spectrum antibiotics. Fifteen days later, she returned for partial intestinal obstruction and pseudocyst infection (purulent contents were aspirated). Two additional plastic stents were placed by ERCP and intravenous antibiotics were administrated. She was discharged with favorable clinical progress (Figures 7–9).
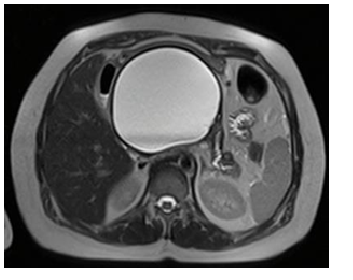
Figure 7 MRCP before drainage: (T2 weighted image). Pseudocyst in the body of pancreas (with minimal detritus) that produces dilation of the main pancreatic duct in the tail and mechanical effect on gastric lumen.
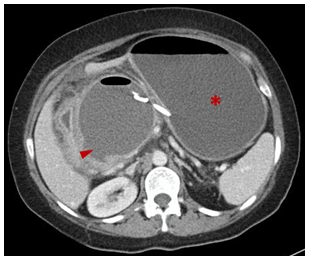
Figure 8 Contrast-enhanced CT (4 weeks after drainage). Pigtail stents between dilated stomach (asterisk) and pseudocyst (arrowheads).
Case 4
A 67-year-old patient with a history of acute biliary pancreatitis (treated in another hospital), cholecystectomy + drainage of gallbladder abscess and arterial hypertension. She presents 7 months later, complaining of abdominal pain and obstructive symptoms that began 7 days after withdrawal of percutaneous drainage of per pancreatic pseudocyst. Physical examination: upper abdominal distention and painful palpation. No alterations in laboratory results. MRCP revealed a per pancreatic pseudocyst of 5.5 cm of diameter in the pancreatic neck, that was punctured with a 19G needle and one double Pigtail stent was placed only because of initial guidewire displacement. After 3 months of drainage, there was clinical resolution and stent was removed (Figure 10).
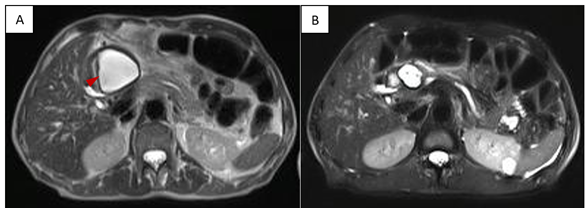
Figure 10 MRCP (T2 weighted image). A. pseudocyst prior to drainage (arrowhead). B. resolution of pseudocyst (3 months after drainage).
Case 5
A 62-year-old woman with a history of cholelithiasis and acute biliary pancreatitis managed in a private clinic (severity not specified). She comes 7 months later for abdominal pain and obstructive symptoms (episodes of nausea and post - prandial vomiting). Abdominal palpation revealed pain in epigastrium. Laboratory results: alkaline phosphatase: 422 IU / L. MRCP: walled-off necrosis of 14 x 12 cm in the pancreatic body and tail. EUS: hypoechoic lesion of 10 x 8.3 cm with a solid content of 30 - 40%. Under fluoroscopic and endoscopic guidance, it was punctured with a 19G needle and 2 double Pigtail stents were left. Due to extensive bleeding in the stomach by puncture of vessels in the gastric wall, the patient underwent an urgent exploratory laparotomy and transfixing points at the site of puncture. No perforation was evident. He had a favorable evolution and was discharged (Figures 11&12).
The drainage of the inflammatory collections can be done by a percutaneous (interventionist radiology), surgical (conventional or laparoscopic) or endoscopic approach, being the last one of first choice and usually performed after 4 weeks of evolution because procedure associated complications are reduced with a mature wall of the collection. A study of 242 patients found that mortality decreased as the intervention (endoscopic, percutaneous or surgical) was delayed more from hospital admission in patients with necrotizing pancreatitis (0 - 14 days: mortality rate of 56%, 14 - 29 days: 26% and > 29 days: 15%, p <0.01).7
Indications for pseudocysts drainage are: presence of symptoms such as abdominal pain, partial or complete obstruction of the gastrointestinal or biliary tract and others, continuous growth and infection.8 As for necrotizing pancreatitis, indications are usually infection with clinical deterioration or persistent organic failure (for several weeks after the onset of acute pancreatitis) without infection or symptomatic collection (gastrointestinal or biliary obstruction);9 while the absolute contraindications for endoscopic drainage are non-encapsulated collections (although there are reports of intervention in these ones), collections located more than 1 cm away from the gastrointestinal tract and the presence of pseudoaneurysms.10
Regarding endoscopic therapy of pseudocysts, the approach may be transmural (most used), Trans papillary or combined. This will depend on four factors: anatomical relationship of the collection to the stomach or duodenum, presence of ductal communication, cyst content and collection size.4
In the transmural technique, which has been used in our case series, drainage is done through the creation of a communication between the pseudocyst and the duodenal or gastric wall. Two biliary plastic stents (double Pigtail) are generally placed; although fully covered self-expanding biliary metal stents (SEMS) are also being used. Recently, was including one specifically Lumen Apposing Metal Stent (LAMS) designed for drainage of pseudocysts (Hot Axios™).11–13 The diameter or number of plastic stents used does not appear to be associated with the number of interventions required for the resolution of uncomplicated pseudocysts.14 Although the advantage of SEMS is that one is sufficient for drainage, there is no evidence to demonstrate the superiority of SEMS over plastic stents for resolution of pancreatic fluid collections.15 Because the risk of migration is greater with metallic stents (with the exception of the Lumen apposing metal stent) some endoscopist prefer to place inside of this 2 double Pigtail stents as an anchor.12
The drainage of walled-off necrosis under the transmural approach is similar to that of pseudocysts; nevertheless, the techniques are more complex and the post-procedure management after initial drainage is more extensive.16 The nasocystic drainage associated with the placement of two transmural Pigtail stents have been traditionally performed to facilitate the evacuation of necrotic remains and improve the rate of success. If clinical improvement with nasocystic lavage and transmural drainage is not achieved after 48 - 72 hours, some authors suggest transmural endoscopic necrosectomy (multiple sessions performed every 48-72 hours to achieve complete removal of the necrotic remains).9,17 Several sites of endoscopic drainage or percutaneous drainage associated can be performed. This combination therapy has been shown to avoid surgical necrosectomy18 and is not associated with pancreatic-cutaneous fistulas or procedure related deaths.
The multiple transluminal drainage technique is another modality, which demonstrated a higher clinical success rate compared to those who received conventional drainage in a study of 60 patients with WON modality.19
A small unicentric study suggests that performing direct endoscopic necrosectomy at the time of initial drainage and stent placement may lead to higher resolution rates, shorter hospital stay and decreased use of health care when compared to the stepwise approach for WON.20
It is important to define prior to the intervention the type of collection that we are facing, since the response to endoscopic therapy is different: 86 - 100% in pseudocysts as opposed to 63 - 81% when walled-off necrosis is treated. Most frequent complications associated with these endoscopic procedures are hemorrhage, perforation, post-procedure infection, and stent migration. In a case report in our country, tension pneumoperitoneum was documented following pseudocyst transgastric drainage guided by endoultrasonography.21
In the GEPARD study,22 complications from endoscopic necrosectomy (n=93) occurred in 26% of patients with a mortality rate of 7.5% at 30 days; while in the Japanese study JENIPaN,23 the rate of complications from endoscopic necrosectomy was 33% with a mortality rate of 11%. In general, the rate of complications is much lower with endoscopic drainage of pseudocysts compared to the drainage of walled-off necrosis.24
Ruiz J et al.,25 described 12 patients who underwent endoscopic drainage of pancreatic pseudocysts in our country demonstrated a success rate of 91.7%.25 This report is the first cases series in Peru of endoscopic drainage of pancreatic collections guided by USE, in which we have seen favorable technical success in all patients and clinical and radiological success too. We had two procedure-associated complications: one patient return to emergency room with WOPN infection, and was resolved with endoscopic drainage plus antibiotic therapy; a second patient (old age patient) who presented gastric hemorrhage that need cistogastrostomy closure by surgical management and stents were removed.
Endoscopic drainage guided by USE is a safe technique for the management of this type of pancreatic and per pancreatic collections; nevertheless, more prospective clinical studies and evidence are needed in our country to determine success and complications rates in this type of therapy.
None.
Authors declare that there is no conflict of interest.
None.

©2017 Sinagra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.