eISSN: 2373-6372


Case Report Volume 5 Issue 5
1Department of Pathology, Apollo Gleneagles Hospital, India
2Department of Neurosurgery, Apollo Gleneagles Hospital, India Department of Oncomedicine, Apollo Gleneagles Hospital, India
Correspondence: Amrita Chakrabarti, Department of Pathology, Apollo Gleneagles Hospital, Kolkata, India
Received: July 28, 2016 | Published: November 17, 2016
Citation: Chakrabarti A, Khan EM, Mishra SK, Quadri A, Ghosh I (2016) Isolated Metastatic Hepatocellular Carcinoma Masquerading as a Pituitary Macroadenoma- A Case Report and Review of Literature. Gastroenterol Hepatol Open Access 5(5): 00155 DOI: 10.15406/ghoa.2016.05.00155
Hepatocellular carcinoma (HCC) is one of the highly malignant and frequent cancers in the world, with a high incidence of extrahepatic metastasis. However, HCC metastasizing to the brain is rare, and isolated involvement of the pituitary and skull base even more infrequent. Such cases may initially present as pituitary adenomas. Differentiation between primary pituitary tumors and metastatic masses is usually done on the basis of clinical presentations, with adenomas commonly being associated with visual field defects and anterior pituitary dysfunction, whereas metastatic masses usually being accompanied by diabetes insipidus (DI), although few cases of cranial nerves involvement and visual defects being the initial manifestation have been reported. Here we describe an extremely rare incidence of metastatic HCC to the sellar and infrasellar region mimicking a pituitary macroadenoma with initial presentations of diplopia, right eyelid drop, generalised body weakness and severe headache as a result of intracerebral bleed with compression of the right 3rd cranial nerve. There were no other signs and symptoms suggesting liver being the primary tumor site. Magnetic resonance imaging (MRI) of the brain was suggestive of a pituitary macroadenoma and the patient subsequently underwent endoscopic decompression and biopsy of the mass. Histopathology and Immunohistochemistry (IHC) of the tumor were diagnostic of metastatic HCC. Follow up investigations revealed the primary liver tumor with no other sites of metastasis. The patient was put on Sorafenib followed by whole brain radiation therapy (WBRT), which resulted in transient improvement, but eventually he succumbed to his rapidly progressive disease.
Keywords:Metastatic hepatocellular carcinoma, Pituitary macroadenoma, Brain metastasis, Oculomotor nerve palsy
HCC, Hepatocellular Carcinoma; DI, Diabetes Insipidus; MRI, Magnetic Resonance Imaging; IHC, Immunohistochemistry; WBRT, Whole Brain Radiation Therapy; FNAC, Fine Needle Aspiration Cytology; PET, Positron Emission Tomography
Tumors in general metastasizing to the pituitary gland and skull base are a rare occurrence, comprising of 1‒3% of all pituitary masses.1 Moreover, breast and lung tumors are the commonest primary malignancies that result in pituitary gland metastasis, with a pituitary mass often being the first manifestation of an occult primary tumor.2 HCC is a frequently occurring malignancy usually manifesting in the 6th and 7th decade of life, with about 64% cases demonstrating extrahepatic metastasis.3 The commonest sites of HCC metastasis include the lungs, regional lymph nodes, kidney, bone marrow and adrenals.3,4 However, brain metastasis is infrequent. Moreover, pituitary and skull base metastasis of HCC has rarely been reported in literature, especially without involvement of any other site. Such isolated cases of pituitary metastasis may closely mimic a pituitary adenoma, however, they usually manifest primarily with DI. Differentiation between a metastatic mass and a pituitary adenoma is usually done on the basis of such clinical presentations where pituitary adenomas are usually associated with visual field defects and anterior pituitary dysfunction, as opposed to DI being the primary presentation of symptomatic metastasis.1,5 However, few cases of pituitary and skull base metastasis have been reported in literature which initially presented with features cranial nerve palsies,1,6 visual field defects7 and hypopituitary symptoms including panhypopituitaris.8 Nevertheless, majority of such metastatic tumors are clinically silent or too small to be diagnosed radiologically. Moreover, when symptomatic, they may have varied clinical and radiological presentations such as tumors, abscesses, cysts, aneurysms, granulomas, trauma or apoplexy; making it difficult to distinguish them from other sellar area lesions.2 The deceptive nature of these metastatic lesions is particularly of significance when there is no clinical evidence of the primary disease, with neurological or endocrinal symptoms being the only manifestation. In such cases, it is essential to correctly diagnose these lesions in order to plan appropriate management strategies. Our case describes that of a 62 year man with isolated cranial metastatic HCC who initially presentated with 3rd cranial nerve palsy, severe headache, and generalized weakness with no other signs or symptoms of primary hepatocellular malignancy. MRI brain was suggestive of a pituitary macroadenoma with subsequent biopsy, histopathological and immunohistochemical analysis of the sellar mass revealing the unlikely diagnosis.
A 62 years old male presented to neurosurgery with a two month history of drooping of right eye lid, generalised body weakness and severe headache. Examination was significant for right sided ptosis and diplopia. History was unremarkable with no significant past illness and there was no clinical evidence of icterus, hepatomegaly or significant abdominal distension. Routine initial investigations including complete blood counts, liver and renal function tests were normal. Subsequently, an MRI brain was done, which was suggestive of a pituitary macroadenoma, showing lobulated mass in the sellar and infrasellar region involving the sphenoid and clivus, with intracerebral bleed compressing the right 3rd cranial nerve (Figure 1). Subsequently, endoscopic decompression and biopsy were performed. The procedure revealed a highly vascular and invasive tumor eroding the sella and sphenoid sinus, suggestive of an invasive macroadenoma.
Histopathological evaluation of the specimen revealed tumor tissue composed of polygonal cells in sheets and trabeculae with hyperchromatic, pleomorphic nuclei, prominent nucleoli and abundant foamy cytoplasm separated by a sinusoidal network and areas of haemorrhage (Figure 2). With the morphological features suggestive of a metastatic HCC, IHC panel was done for confirmation. Glypican 3 showed strong positivity (Figure 3), along with cytokeratin, while the other markers including synaptophysin, S‒100, EMA, and vimentin were negative, confirming hepatocellular origin of the tumor.
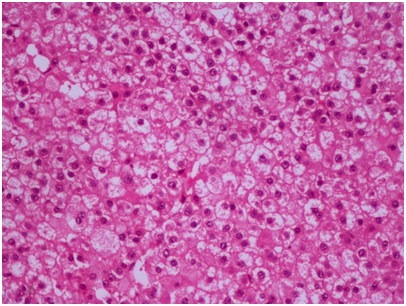
Figure 2 Tumor tissue composed of polygonal cells in sheets and trabeculae with hyperchromatic, pleomorphic nuclei, prominent nucleoli and abundant foamy cytoplasm (H&E 20X).
Meanwhile, further diagnostic workup was done, with abdominal ultrasound revealing a 5.5x3.9cm mass in the left lobe of liver, multiple portal nodal enlargement and moderate ascites. A liver biopsy from the mass was performed, which was confirmed to be HCC. FNAC of enlarged portal nodes were reactive in nature. Whole body PET scan showed no other metastatic focus. Alpha fetoprotein was found to be 119.5ng/ml, serum alkaline phosphatase 208U/L, SGPT 61 U/L, SGOT 115 U/L and a total bilirubin of 1.2 mg/dl. HbsAg and Anti HCV were negative.
The patient was started on low dose Sorafenib (200mg twice daily) in view of Child‒Pugh B and poor performance status. Eventually, WBRT was administered owing to worsening neurological symptoms, while resulted in mild and transient improvement. The patient died after 4 weeks of initiating radiotherapy.
Isolated metastatic involvement of the pituitary and skull base is an uncommon presentation of HCC. A study by Katyal et al.4 demonstrated only 3 out of 148 cases of HCC with brain metastasis, with all 3 at the gray‒white matter junction and being accompanied with metastasis at other sites. Shuangshoti et al.9 stated that metastatic HCC comprise of only 1.3 to 2.9% cases of intracranial metastatic tumors. Further, Morita et al reported only one case of HCC among 36 surgically resected pituitary metastases.10 Likewise, few other reports are available in literature describing HCC with intra cranial metastasis. However, most of such cases are accompanied by metastases at other sites like lung or bones. Moreover, among cases of brains metastasis, pituitary and skull base are even rarer sites of involvement, with most such cases being recognized in autopsy specimens due to very infrequent clinical presentations.1 In the presence of clinical manifestations, diabetes insidipus is the commonest presentation due to direct blood flow from the systemic circulation into the posterior lobe.1,10 Though other symptoms such as headache, hypopituitarism, visual field defects and cranial nerve palsies have also been reported, these features primarily suggest a pituitary macroadenoma, especially in the absence of clinical features suggestive of the primary malignancy and no other sites of metastatic involvement. Few rare case reports include that of Trivedi et al.6 & Tamura et al.1 who report 1 and 2 such case of isolated skull base metastasis of HCC primarily presenting with cranial nerve palsies and extraocular symptoms respectively.
Metastasis in and around the sella occurs mainly via direct hematogenous spread to the pituitary parenchyma or diaphragm sellae, spread through the portal vessels, extension from juxtasellar and skull base metastasis, or meningeal spread through the suprasellar cistern.2 The posterior lobe, being supplied by hypophyseal arteries, is most commonly involved, comprising about 84.6% cases whereas anterior lobe is affecting only 15.4% cases; thus explaining DI being the commonest presenting feature. As per McCornick et al.11, of 40 symptomatic cases, 70% had DI, whereas only 15% cases had features of anterior pituitary insufficiency. Hypopituitarism and rarely, hyperfunctional syndromes including Cushing’s syndrome and acromegaly have also been reported.2 Bilateral hemianopsia is the commonest type of visual impairement, with infiltration of the cavernous sinus resulting in oculomotor nerve palsy.
Radiological evaluation is in general not very effective in distinguishing pituitary metastasis from adenomas in the absence of other coexisting metastatic brain lesions.2 However, high resolution CT and MRI are more sensitive than other imaging modalities. Nevertheless, confirmation of diagnosis relies on histology. In instances where features of metastasis coexist with that of with adenomas, or adenomas showing marked nuclear pleomorphism, tumor giant cells or brisk mitosis, the role of IHC becomes imperative.12
Prognostically, HCC with brain metastasis is extremely poor. As reported in previous studies, the median survival ranged from 4 to 12 weeks.13 Despite poor prognosis, surgery at the metastatic site may improve symptoms. However, a study showed treatment with WBRT resulted in a longer median survival time compared to patients treated with surgical resection followed by WBRT.13 While surgery may be feasible in cases of metastatic deposits in accessible areas, brain irradiation can prolong the survival along with improving the quality of life.3 Furthermore, Child‒Pugh classification is also considered an important prognostic factor, with few studies highlighting its importance in influencing the medial survival time.14 Likewise, our case was classified as Child‒Pugh B, managed with an initial endoscopic decompression followed by Sorafenib and WBRT. After a brief transient improvement, the patient succumbed after 4 weeks of initiating radiotherapy.
The rarity of our case lies in the fact that this was an isolated skull base metastatic HCC clinically and radiologically presenting primarily with features of a pituitary macroadenoma, with no initial clinical evidence of the primary malignancy and no other site of metastasis.
Although extremely rare, isolated skull base or pituitary metastasis of HCC can occur, and may present deceptively as a pituitary adenoma. Clinical suspicion and diagnosis becomes difficult in such cases, especially if they are not accompanied by signs and symptoms of obvious primary malignancy or other sites of metastatic involvement. Careful clinical assessment and diagnostic workup are essential for the detection and management of such cases in an attempt to increase the median survival time or improve the quality of life in spite of a dismal prognosis.
None.
None.

©2016 Chakrabarti, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.