eISSN: 2373-6372


Research Article Volume 5 Issue 4
1Clinica Internacional, Lima, Peru
2Hospital Nacional Edgardo Rebagliati Martins and National University of San Marcos, Lima, Peru
Correspondence: Julio Francisco Leon Moreno, Clinica Internacional, Lima, Peru, Tel 51-949356871
Received: August 30, 2016 | Published: October 7, 2016
Citation: León JF, Díaz JO (2016) Fasciola Hepatica Infections: Differences in the Clinical, Analytical and Imaging Assessments. GastroenterolHepatol Open Access 5(4): 00148. DOI: 10.15406/ghoa.2016.05.00148
We present the cases of three patients from different rural areas of Peru whose clinical assessments indicated abdominal pain as a main symptom, and who showed associated eosinophilia. There were also various findings through imaging, especially CT scans (e.g. single or multiple hypodense abnormalities, subcapsular hematoma), which led to different diagnosis in each case. Serologic tests (ELISA or Western Blot) on all the patients were performed to obtain a definitive diagnosis. The patients were treated with triclabendazole (“TBZ”)-recommended medication for this disease in Peru-resulting in satisfactory results in all three cases, as confirmed by the clinical, analytical and imaging assessments. Patient tolerance to the medication was noted as good.
Keywords: fasciola hepatica, eosinophilia, triclabenzadole
In 1379 de Brie first described the morphology of the trematode parasite in sheep.1 In 1758 Carl Linnaeus named it Fasciola hepatica, indicating its leaf shape and the organ most affected by it.1 Parasitism in human beings has been identified specifically in the following countries: Venezuela, Uruguay, Brazil, Argentina, Chile, Puerto Rico, Cuba, Mexico, Guatemala, Costa Rica, Syria, Turkey, China, Russia, Poland, Portugal (Madeira), England, France, Italy, Somalia, South Africa and the United States, including Hawaii.1
Fasciolasis is a cosmopolitan parasitic zoonosis produced by the trematode Fasciola hepatica in its adult stage, that affects herbivorous mammals (cattle, goats, sheep) and occasionally human beings as incidental hosts.2. It is considered a reemerging tropical disease, endemic in Latin America and also a public health problem in Peru, Bolivia, Chile and Ecuador.3. In Peru, the prevalence has been estimated in the following rural areas, to be: 34% of the sample population in the Mantaro valley,4,5, and 0.4 to 40% in Cajamarca, depending on the locality.6
Fasciola hepatica is a non-segmented flat hermafrodite trematode, measuring 2-3 cm long by 10-13 mm wide.7. Human beings are infected by consuming aquatic vegetables contaminated with metacercarias (laval form encysted and resting), particularly wild watercress (nasturdium officinale), besides mint, alfalfa, reeds, lettuce and spinach. Other sources of infection are: ingesting badly cooked liver of infected animals and, on a lesser scale, drinking contaminated water.
The biological cycle can be in one of two forms:
Case no. 1
The patient was a 44 year-old woman from a rural coastal area born in and resident of the town of Barranca (Department of Lima) where she works as a domestic servant. She was found to have no other pre-existing contributory factors to her clinical condition which had began two months prior to assessment. She presented abdominal pain on the flank and right hipochondrium, colic pains, of variable intensity, un quantified weight loss, but no other discomforts. She was assessed at the Outpatients Department and on medical examination, it was noted-on superficial as well as deep palpation-that there was pain on the flank and on the right hypochondrium, there were no associated signs of peritoneal irritation. To assist with the diagnosis, tests were carried out Table 1 which showed anemia with an increase in the white blood cells count and eosinophilia (32%). On the hepatic profile a slight increase in alanine aminotransferase (ALT) and alkaline phosphatase (ALP), with the bilirubin within normal values: the c-reactive protein (CPR) was found to be high (64.69 mg/dl). The parasitological tests were negative. The abdominal ultrasound concluded there was the presence of a hyperechogenic area of badly defined borders at the level of the right hepatic lobule compromising segments VI and VII. The rest of the assessed structures had a conservative morphology. The study was complemented with an abdominal CT scan (Figure 1). Tests for antibodies against Fasciola hepatica (indirect ELISA) resulted positive.
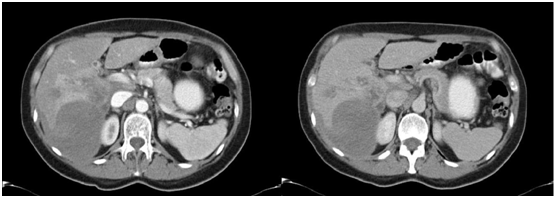
Figure 1 Abdominal CT scan with contrast in arterial and venous phases showing the presence of a hypodense image in the right hepatic lobule (RHL) compromising segments VI and VII suggesting subcpasular haematoma.
Normal value/Range |
Case no. 1 |
Case no. 2 |
Case no. 3 |
|
Hematocrit (%) |
35/38 - 52 |
32 |
34.72 |
31 |
Hemoglobin (g/dl) |
11.5/12.5 - 16.5 |
10.3 |
11.2 |
10 |
Leukocytes (per mm3) |
4 000 – 10 000 |
13340 |
4630 |
20900 |
Neutrophils (%) |
55– 75 |
42 |
34.1 |
25 |
Lymphocytes (%) |
20-35 |
19 |
51.4 |
23 |
Monocytes (%) |
03-10 |
7 |
7.5 |
2 |
Eosinophils (%) |
01-5 |
32 |
6.9 |
49 |
Basophils (%) |
0-2 |
0 |
0.1 |
1 |
Platelets (per mm3) |
150000-450000 |
240000 |
204000 |
551000 |
C-reactive protein (mg/dl) |
0-0.5 |
64.69 |
42 |
13.2 |
Prothrombin time (sec) |
9.4-12.5 |
13.3 |
12.3 |
16 |
International Normalized Ratio (INR) |
0.80-1.2 |
1.1 |
1 |
1.25 |
Urea (mg/dl) |
19-40 |
35 |
26 |
28 |
Creatinine (mg/dl) |
0.7-1.2 |
0.7 |
0.83 |
0.45 |
Glucose (mg/dl) |
70-110 |
87 |
78 |
64 |
Total Bilirubin (mg/dl) |
0.3-1.2 |
0.88 |
2.41 |
0.4 |
Direct Bilirubin (mg/dl) |
0.1-0.3 |
0.29 |
0.47 |
0.2 |
Indirect Bilirubin (mg/dl) |
<0=0.9 |
0.59 |
1.94 |
0.2 |
Aspartate aminotransferase (U/l) |
9-37 |
34 |
40 |
121 |
Alanine aminotransferase (U/l) |
9-43 |
44 |
80 |
104 |
Total Protein (g/dl) |
6.5-8.1 |
8.27 |
6.6 |
7.9 |
Albumin (g/dl) |
3.5-4.8 |
4.09 |
4.24 |
4.1 |
Alkaline phosphatase (U/l) |
60-300 |
394 |
230 |
846 |
Gamma-glutamyl transpeptidase (U/l) |
7-50 |
40 |
85 |
63 |
Parasitology test (faeces) |
Negative |
Negative |
Negative |
Negative |
Alpha-fetoprotein (UI/ml) |
0-7 |
7.48 |
4.01 |
- |
Antibodies against Fasciola hepatica (indirect ELISA) |
<0.2:no infection |
1.84 |
- |
- |
Western Blot for Fasciola hepatica |
Negative |
- |
Positive |
Positive |
Western Blot for Hydatid cyst |
Negative |
- |
Negative |
|
Hepatitis Serology (Hepatitis B Virus, Hepatitis C Virus) |
Non-reactive (NR) |
NR |
NR |
NR |
Table 1 Results of Daignostic Tests
Case no. 2
The patient was a 33 year old man from a central highlands rural area born in and resident of the town of Huancayo (Department of Junin) where he works as an administrative clerk. There were no other contributory pre-exisiting factors to his clinical condition which began 45 days prior to assessment. He presented abdominal pain, sharp and piercing in nature, in the epigastirum and right hypochondrium. He was treated with the proton pump inhibitor (Pantoprazole) but without showing any subsequent clinical improvement. An upper digestive endoscopy was performed, showing superficial cronic gastritis resulting negative for Helicobacter pylori. An abdominal ultrasound scan showed the presence of hepatic nodules in segments VI and VII. Additionally, CT scans of the abdomen and pelvis showed hepatomegaly and a hipodense lesion compromising liver segments VI, VII and VIII. The lesion was highlighted by the use of contrast in subsequent phases and the retroperitoneal and inguinal lymph nodes were not significant. To further clarify the clinical condition, an MRI abdominal scan (Figure 2) was performed which suggested micro hepatic abscesses but remaining alert to the possibility of a malignant neoplasm. Additional tests (Table 1) indicated slight anemia with hemoglobin at 11.2 gr/dl, hematocrite 36%, white blood cells count 4630/mm3, increase in eosinophils 6.9%. Hepatic profile with slight increase in transaminases, a predominance of alanine aminotransferase (ALT), with bilirubins within normal values. The parasitological evaluation of faeces was negative. Alpha-fetoprotein and carcinoembrionary antigen within normal values. Western Blot for hydatidosis was negative. However, Western Blot and antibodies for Fasciola hepatica were positive.
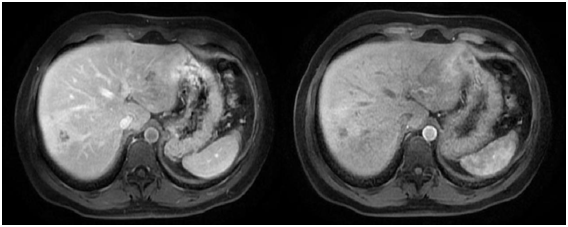
Figure 2 Abdominal MRI with contrast showing the presence in the liver of badly defined images with irregular borders, with hypointense centre and periferic enhancement after the application of the contrast in segments VI, VII and VIII suggesting micro abscesses.
Case no. 3
The patient was a three-year old male child from a northern highlands rural area born in and resident of the town of Cajamarca (department of Cajamarca). His was a eutocic birth without complications and there were no other pre-existing contributory factors of his clinical condition which began 20 days prior to his assessment. He presented fever, nausea, and irritability, and later developed abdominal pain in the superior right quadrant and an increase in his abdominal girth. He was treated with paracetamol. Temperature 38.5˚C, respiratory rate 24/min, heart rate 110/min, mildly pale of aspect, oedema in lower limbs +/+++. Distended abdomen. Diffuse pain with superficial and deep palpations. Liver palpable 6 cm below the right ribcage edge. Indications of ascents. Abdominal girth 63 cm. Diagnostic tests (see Table 1) highlighted slight anemia with hemoglobin 10 gr/dl, hematocrite 31%, high white blood cell count, and eosinophilia 25%. Hepatic profile with elevated aminotransferases with a predominance of alanine aminotransferase (ALT), bilirubins within normal values, prothrombin time prolonged, alkaline phosphatase (ALP) elevated. Parasitological evaluation was negative. Serology tests for hepatitis A, B and C were negative. Ascitic fluid analysis showed elevated leucocytes with a predominance of polymorphonuclear ones (85%), ADA test and culture negative. Hemoculture negative. The abdominal ultrasound revealed hepatomegaly with heterogenous echotexture diffusely in the hepatic parenchyma. Fusiform and ovoid images 7x2 mm in the LHL and discreet focal dilatation of the biliary duct. Spleenomegaly, ascites, bilateral pleural effusion. Abdominal CT scan (Figure 3) revealed a normal liver as regards shape and position, smooth surface and sharp borders. However, the liver was enlarged with multiple hipodense diffuse heterogeneous abnormalities, associated with a narrowing of the suprahepatic veins on the right side without focal mass effects. There was also pleural effusion in both pulmonary bases, slight hepatospleenomegaly and ascites; all of these suggested the Budd Chiari Syndrome. Immunoblot IgG for Fasciola hepatica was positive (presence of 02 diagnostic bands).
Fascioliasis hepatica is a global infectious parasitic disease caused by the trematodes Fasciola hepatica or Fasciola gigantica. The former one finds in Europe, Asia, the Middle East and Latin America. The latter has been reported in Asia, Africa and Hawaii.4
In recent years it has been considered an infection of significant impact in human beings, with approximately 17 million people affected in 51 countries,4 and a serious public health problem in Peru, Bolivia, Chile and Ecuador.3 It affects herbivorous such as cattle, pigs, horses, rodents, as well as others, and also human beings. In Peru various studies have been undertaken on animals and human beings using serological and coprological methods that have demonstrated the presence of these parasites, even up to hyperendemic levels, in the valleys of the highlands.16–18
As regards the prevalence of the infection, the highest levels in Latin America are found in the highlands.4 In Bolivia it has been reported that the rate of prevalence of the disease has been up to 90%. In Peru it has been reported in recent years that there is a larger number of cases of infected humans than in other regions.10,19–21
The Fasciola hepatica infection is one of the three most important zoonoses that affects the human liver in Peru.22 In 2002 in the altiplano (highlands) peruano the global prevalence of the infection was 24.3 % (reported by using coproparasitological methods).23 In the same area two years later the prevalence was found to be 71.4% of serological positives using Fas2ELISA.19. Other areas of Peru where the Fascioliasis is present with high prevalence rates includes: the Mantaro Valley (up to 36%),24 Cajamarca (8%),25 and Huarochiri (up to 36%).19,26 In 2007 Marcos LA et al.16 published a paper in which it was emphasized, among other points, that the number of cases of infected persons that were reported in the period 1963 to 2005 in Peru had all occurred in 17 Departments out of a total of Peru's 24 Departments. The Departments affected were: Abancay, Amazonas, Ancash, Apurimac, Arequipa, Ayacucho, Cajamarca, Cuzco, Huancavelica, Huanuco, Ica, Junin, La Libertad, Lima, Moquegua, Tacna and Puno. The report highlights that 71% of the Peruvian Andean Highlands territory would be affected by this zoonosis.16
As regards the three patients of our study presented here, they all come from Departments that are mentioned in the publication above16 and live in rural areas. Their places of residence have a degree of relationship with the epidemiological characteristics required to complete the biological cycle and the transmission of the disease (ambient temperature and humidity, reservoirs of water and lakes and other standing water, viability of the intermediary host, dietary habits, and infected animals).27
There exist reports in which it is mentioned that the infections occur with higher frequency in relation to: males, certain occupations, dietary and recreational habits. However, this did not occur in our patients as mentoned in other publications.9,28,29
Fasciola hepatica is a trematode in the shape of an elongated leaf measuring approximately 20 to 30 mm long and 10 to 15 mm wide. In its adult stage it locates itself in the biliar tract of cattle, sheep or goats and, accidentally, in humans. The trematode has in the middle of its body a ventral sucker that allows it to fix itself to the walls of the biliar ducts. It is hermaphrodite, self fertilizes and, after a period of time, can lay 600 eggs a day. The eggs form an embryo in fresh water, and they develop a larval form with cilia or miracidium, which mature in 15 days, leave the water and swim until they find fresh water snails (principally of the genus Lymnae). Inside the snails they transform into a sporocyst and later in redia mother and redia daughter and, within these, the cercaria are formed. These abandon the snail and swim to encyst themselves in semi-submerged aquatic plants (watercress) or in the bottom of slow moving water forming metacercarias that are infectious to humans.30 In our studies, two of our three patients (from Cajamarca and Huancayo) consumed watercress, and the other patient did not really know what watercress was, therefore he could not confirm or deny whether she had consumed or not. One needs to take into account that the presence of this parasite is associated with the ingestion of watercress in up to 70% of the cases and that it is also found in the other plants mentioned earlier.16
The clinical picture tends to be non-specific, thus generating certain degree of difficulty at the point of determining the scope and nature of diagnostic tests being necessary on many occasions to rely on subsequent additional tests, particularly imaging and serology specific to the causal agent. For all patients presenting non-specific abdominal pain and eosinophilia in the hemogram, we should take into account the epidemiological factors, such as: the geographical area of the patient's residence and its prevalence to Fasciola hepatica and the consumption of aquatic plants, particularly watercress types, to be able to eliminate the possibility of Fasciola hepatica infection.
Fascioliasis is characterized by having two phases (acute and chronic). Determining which of these phases is applicable at the moment of clinical evaluation of the patient is important when interpreting clinical data and the results of diagnostic tests.
The acute or hepatic phase is produced by the migration of the parasite through the patient's hepatic parenchyma, usually accompanied by fever, pain in the right hypochondrium, hepatomegaly and eosinophilia. This occurs 6 to 12 weeks after the ingestion of the metacercaria. One could associate other symptoms, such as: jaundice, nausea, vomiting, myalgia, urticaria, anorexia and cough, or complications such as: haemobilia or hepatic sub capsular hematoma, and in the case of serious infections, extensive hepatic necrosis.30 The symptoms tend to disappear within six weeks. In addition, pleural effusion to the right side with eosinophils may occur.31 Other reactions have also been reported, including: meningeal symptoms, neurological focal changes, convulsions, pericarditis, and abnormalities in cardiac conduction, but these reactions are infrequent. These could probably relate to immunological or allergic mechanisms.32
The chronic or biliar phase is generally characterized as being asymptomatic. However, after the obturation of the biliary ducts by the adult trematodes, biliary colics, cholangitis and obstructive jaundice could result. In addition to the classic symptomatology one could develop further complications, such as: biliary cirrhosis or sclerosing cholangitis. The eosinophilia can present results over a wide range.32
Analyzing the clinical presentation of our three patients, two of them presented abdominal pain without other clinical manifestations. One patient presented weight loss and one presented a broad set of symptoms including: fever, nausea, irritability and, in the physical examination, a mild pale aspect, hepatomegaly and ascites. The focus of diagnostic testing, when presented with patients whose clinical pictures are similar to those mentioned above, will be variable and dependent on the manifestation seen in each patient. In relation to the abdominal pain and the loss of weight, these could have multiple etiologies, since their semiological value is poor. In relation to the male child patient that presented a broad set of symptoms, and noting that he came from Cajamarca where there is a high prevalence of infection by the trematode parasite, the suspicion of a linkage is heightened. In some of the reports, the abdominal pain can present itself in both the acute and chronic phases in approximately 91.8 % and 92%, respectively.33 In the reports of infected humans in Peru between 1963 and 2005, 11% corresponded to cases in the acute phase and 77.1% to cases in the chronic phase, and 9.8% to cases in the chronic asymptomatic phase.16
As regards determining which phase of the disease was applicable at the point of clinical evaluation, all three patients presented abdominal pain but only in the male child patient who presented a broad set of symptoms including fever, could we infer with a certain degree of precision that he was in the acute phase of the disease. For the other two patients it was difficult to be precise as to what phase of the disease they were in even though the period of initiation of symptoms in the two cases was not greater than eight weeks.
However, given the difficulties of aligning the diagnostic process and supporting tests, it remains highly subjective in which phase the patient is. The hemogram shows mild to moderate anemia present in all three patients. It has been reported that leucocytosis with eosinophilia is present in 95% of patients in the acute phase, whilst in the chronic phase these findings are more variable.34 For our patients, two presented with leucocytosis and all three with eosinophilia at the point of evaluation. This variability is a reflection of which phase the patients were actually in at the point of evaluation. There could have been an increase in the reactants of the acute phase (elevated in all three of our patients). Other alterations in the blood analysis could influence the hepatic profile by triggering an increase of the aminotransferases (AST, ALT), an increase of the alkaline phosphatase (ALP) or an increase of the bilirubins, these in relation to the degree of impact on the hepatic parenchyma (hepatocellular injury) or a cholestatic lesion arising from a complication of the infection, or the presence of the trematode parasite in the biliary duct causing a certain degree of obstruction.34 In our patients only two presented a certain increase in hepatic enzymes, a fact that relates directly with the impact on the hepatic parenchyma mentioned previously.
Although the parasitological examination is frequently performed in many centers, one must keep in mind that even if the presence of the trematode parasites in serial faeces examinations confirms the diagnosis, their absence does not exclude it, since the observation of these is related to the phase of the disease (they are not observed in the acute phase). The identification of the eggs can also be done by duodenal aspiration, and again although the presence of the parasites confirms the diagnosis, their absence does not exclude it.34 In our patients, they all underwent serial examinations for parasites in the faeces, none of them resulted positive. In spite of this result, and because of the clinical, analytical and imaging suspicion, the diagnostic studies continued with greater testing precision, sensitivity and specificity, to achieve the definitive diagnosis.
Of the imaging techniques, the CT scan is the most useful, being able to show hipodense nodules or tortuous liver tracts as a result of the parasites migration path, as well as a thickening of the hepatic capsule, a sub capsular hematoma, or parenchymal calcifications.35 The MRI scan can be utilized in certain cases because it permits us to characterize lesions in different patterns, some authors propose that the patterns can be classified in five types (type 1 to type 5). Noting that up to 10% of cases can present a normal MRI and that type 2 is the most frequent with 62.1% (iso or hipointense area in type 1, badly defined hyperintense area in type 2, and enhancement of the capsule with Gadolineum).36 During the chronic phase the ultrasound and the retrograde endoscopic cholangiopancreatography are able to show the displacement of the trematodes through the biliar tract and gall bladder. In some cases one can observe a biliar thickening in the common bile duct. The MRI appears to have no advantages over other imaging techniques.37 All our patients had received imaging during the diagnostic studies (these were performed prior to our evaluation). If one were to value a study by images in this type of patients, one could realize the great variety of presentation forms that must be taken into account at the point of realizing a differential diagnosis.
In our patients, each one has a different image as a result of the varying diagnostic approach (abdominal ultrasound, CT scan or MRI scan). The patient in Case no.1 had two imaging examinations: abdominal ultrasound and CT scan. The abdominal ultrasound indicated the presence of a hyperechogenic area in right hepatic lobule (RHL) that compromised segments VI and VII. The CT scan showed the presence of a hipodense image of well defined borders at the level of segments VI and VII (see Fig. 1) that could correspond to a hepatic abscess. The patient in Case no. 2 had three imaging examinations: abdominal ultrasound, CT scan and MRI scan. The abdominal ultrasound showed the presence of hepatic nodules in segments VI and VII. The abdominal CT scan reported hipodense nodules in segments VI, VII and VIII with contrast medium enhancement at later stages, as well as hepatomegaly. The MRI scan demonstrated images suggestive of hepatic micro abscesses (Figure 2). The patient in Case no. 3 had two imaging examinations: abdominal ultrasound and abdominal CT scan. The abdominal ultrasound revealed hepatomegaly and fusiform and ovoid images on the left hepatic lobule (LHL) and dilatation of the biliary duct. The abdominal CT scan demonstrated multiple hipodense, heterogeneous images, with a diffuse distribution (Figure 3). Analyzing the findings of the imaging examinations of our three patients, despite the multiple formats of presentation, there is agreement of the majority of the findings with those reported by others elsewhere, although the imaging examinations did not permit us to make a definitive diagnosis in a nosological entity sense, in a certain manner the imaging examinations in conjunction with the clinical pictures and the blood analysis permitted us to put aside the possibilities of other etiologies, particularly neoplastic or infectious (hepatic abscesses and other parasitosis, such as hydatidosis).
The election of serological methods for the diagnosis of the acute phase was important because of the absence of eggs of Fasciola hepatica in that phase. Besides, it is worth noting that when infection is not very intense, the coproparasitological examination even in the chronic phase does not usually give satisfactory results.30 It is in this moment where the serological methods are critically important to reach a diagnosis in this entity. Multiple tests have been used for the diagnosis of the Fasciola hepatica infection. The technique Fas2- ELISA has practically replaced other serological tests with a sensitivity ranging between 92% and 98% and a specificity of 83.6% according to other reports.37 In the acute phase Fas2-ELISA has the advantage of not presenting crossed reactions with helminth infections prevalent in areas where Fasciola hepatica is endemic. It is also useful for the screening of populations inhabiting areas endemic to these parasitic infections.37,38 The low specificity of Fas2-ELISA implies an elevated number of false negatives. This has permitted the development and utilization of the 'Western Blot' following the utilization of antigens of excretion-secretion of adult forms of the parasite. Taking into account that the sensitivity of this test can be 95.5% and the specificity up to 100%, it could be considered confirmatory. Our three patients presented positive serological tests for Fasciola hepatica, thus confirming the diagnosis.
The treatment for Fasciola hepatica should be efficient as well as appropriate to prevent irreversible effects that this type of parasite can cause in human beings. In recent years, the preferred medication treatment has been with Triclabenzadole due to its excellent effectiveness and tolerance.39 The usual dose is 10 mg/kg of body weight and there has been no major variation between various authors as regards this dose; however, there are differences of opinion on the number of cycles best applied, whether it should be one, two or even up to three cycles, depending on the results of the coproparasitological tests.30 However, the usefulness of the coproparasitological test is dependent upon which phase the disease is in and the intensity of the infection. It would appear from the published evidence that the dosing regimens fall into one of the following three categories:
(a) 10 mg/kg of body weight administered on day one and, if tests deemed it necessary, a second dose of the same quantity 48 hours later
(b) 10 mg/kg of body weight as a single dose, and
(c) two doses of 7.5 mg/kg of body weight on the same day of treatment.
As regards the efficiency, it appears that the dosage scheme (a) achieves 100%, that of scheme (b) achieves 96%, and that of scheme (c) achieves 100%.16,40,41
Each of our three patients received treatment for Fasciola hepatica with triclabenzadole with one 10mg/kg dose and a second dose after 48 hours. In the two adult patients the triclabendazole medication was for veterinary use, whereas for the male child patient the triclabendazole medication was specially prepared by a Veterinary Doctor in cooperation with the Clinical pharmacist.
One can be confident that the Fasciola hepatica infection has been erradicated once the post-treatment serological tests show negative. In our patients the serological tests were carried out on a monthly basis and negativization occurred between the second and fourth month. However, we recommended our patients to have additional tests in order to assess the clinical status of their condition to confirm: the disappearance of eosinophilia, a faeces test for Fasciola hepatica eggs and imaging tests42 to ensure no long-term effects of the disease. With respect to our three patients' post-treatment, they all showed an improvement in the clinical picture and a reduction of the eosinophilia. They also embarked on a program of imaging checks at 6 month intervals, which confirmed there were no long-term effects of the disease.
We have presented case studies for three patients of different sex, age, place of residence, who all presented varying clinical pictures, but notably all of them presented abdominal pain and the blood analysis showed eosinophilia initially and before starting treatment. It is worth noting that the differential diagnostic bases on the different imaging examinations were different in all cases, due to the particular characteristics of each patient. The clinical findings of the blood and imaging analysis were in agreement with the findings reported elsewhere in the medical literature. All three patients showed good tolerance of the medication and a good response to their treatment.
Fasciola hepatica infections are a serious public health problem in Peru because the geographical areas at high risk represent some 70% of the Peruvian territory (Andean Highlands) and, secondly, because the prevalence of Fasciola hepatica infections is increasing in these areas. The Fasciola hepatica transmission chain is well documented with 70% of infections related to the consumption of watercress by persons living in the high risk areas. Consequently, when undertaking the clinical evaluation of patients from these high risk areas with abdominal pain and eosinophilia, one should consider the possibility of a Fasciola hepatica infection and, for the clinical evaluation, include questions of the patient's food consumption habits as well as performing the relevant faeces tests and Fasciola hepatica tests. Once the presence of Fasciola hepatica infection has been established and successfully treated, then it is also important to ensure long term follow-up checks to control for any possible long term effects, possibly irreversible, that could have arisen from the Fasciola hepatica infection.
None.
The authors declare no conflicts of interest.
None.

©2016 León, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.