eISSN: 2577-8307


Research Article Volume 3 Issue 4
1Federal University of Piaui, Brazil
2Federal University of Vicosa, Brazil
Correspondence: Marlete Moreira de Sousa Mendes, Universidade Federal do Piauí, Campus Professora Cinobelina Elvas, Brazil, Tel +00-55-89-99332627
Received: September 18, 2019 | Published: November 28, 2019
Citation: MMDS, Lacerda CFD, Fernandes FEP, et al. Nutrient input and output in an agro forestry system in a semiarid region of Brazil. Forest Res Eng Int J. 2019;3(4):146-152. DOI: 10.15406/freij.2019.03.00091
Alternatives to traditional agricultural farming practices that combine production and environmental conservation, such as agroforestry systems, are currently being studied. This study aimed to quantify the contribution of trees to nutrient input and output in crops grown in an agrosilvopastoral system in the municipality of Sobral, Ceará State, Brazil. Nutrient concentrations were quantified in shade and sun leaves of Cordia oncocalyx Allemão (called pau-branco) trees in the rainy and dry seasons and in maize leaves at the time of harvest. Nutrient concentrations in different soil layers (0-10, 10-20 and 20-40cm) were also quantified at 0.4 and 4.0 m from the trunk of C. oncocalyx trees. The contribution of the trees to the nutrient input to the system and nutrient output due to the removal of maize plants were also assessed. The soil under the canopy of C. oncocalyx showed the highest concentrations of total N, K, P, Fe, Cu, Zn and Mn. However, few differences were noted in the concentrations of maize leaf nutrients as a function of the distance from the trunk. The trees may contribute up to 35kg ha-1 Ca, 19kg ha-1 N and 15kg ha-1 K, whereas approximately 2.3kg N, 5.6kg K and 0.2kg Ca leave the system with the removal of maize plant shoots. Therefore, maintaining trees in production systems significantly contributes to replenishing the nutrients lost from harvesting crops.
Keywords: cordia oncocalyx, agrosilvopastoral system, maize, nutrient cycling
AFS, agro forestry systems; CEC, cation exchange capacity; TN, total N; ASP, agrosilvopastoral system; TDM, total dry mass ; TPS, total concentration per system; TPT, total per tree
Mineral nutrients are important because deficiencies preclude plants from completing their life cycle or developing, and they reach the soil through weathering, organic matter mineralisation, atmospheric deposition,1 runoff from rainfall that leaches minerals from leaves and stems,2 or even through fertilisation. Fires, erosion, leaching and vegetation removal are among the methods by which soil loses mineral nutrients. Thus, the management of agricultural systems should include an appropriate balance between inputs and outputs, where losses are minimal and limited to the harvest of the marketable product to maintain soil fertility.
Agro forestry systems (AFS) have emerged as an alternative agricultural practice because they enable the maintenance of native or exotic tree/shrub species in cultivated areas and are based on the premise that land-use systems that are structurally and functionally more complex than monocultures result in increased efficiency in the capture and utilisation of environmental resources (nutrients, light and water).3 Studies have shown that AFS may decrease N leaching and increase C immobilisation,4 as well as the pH, cation exchange capacity (CEC), exchangeable bases, concentrations of N, P and K,5 and soil organic C (SOC).6
Nevertheless, studies comparing AFS with other types of land use are inconsistent, and AFS is not indicated as a key promoter of soil fertility maintenance and/or increase by all researchers. Researchers,7 recorded similarities between the SOC concentrations in forests and AFS with cacao cultivation, whereas others,8,9 observed lower SOC concentrations in an AFS than in a forest that was associated with high C losses because of erosion. No differences in SOC concentrations was observed between conventional tillage systems and AFS with subsistence crops in Nepal.10 In addition, the use of AFS in the Atlantic region of Costa Rica produced lower concentrations of exchangeable bases than pastures, whereas the total N (TN) and SOC concentrations did not differ.11 Soil disturbance in an agrosilvopastoral system (a type of AFS combining crops and livestock maintaining trees) in a semiarid region caused a reduction in SOC concentrations,12 furthermore, the concentrations of Ca2+ and Mg2+ were higher in some layers in areas under traditional cultivation and in forests used as a reference.
AFS components include trees, crops and animals, and the effect of AFS on the development of trees or crops has been reported in the literature.13–16 There is evidence that agro forestry management may provide beneficial effects for crops compared with crops grown in monoculture.17,18 Conversely, agro forestry management may negatively affect the development of the trees because of competition with crops.19
Management systems that include vegetation removal are known to change the nutrient dynamics of an area because (i) bare soils tend to lose nutrients by surface runoff, (ii) a decreased number of tree species produces less litter, which reduces soil nutrients, and (iii) the export of removed plant parts reduces plant nutrients. Conversely, the effect of agro forestry management on the nutrient dynamics of the system is not well known, especially in the Brazilian semiarid region. Therefore, this study aimed to quantify the contribution of trees to soil nutrient input and output with the harvest of crops and assess the effect of trees on the nutrient concentrations of crops.
Study area
The experimental area is located at Crioula Farm, which belongs to Brazilian Agricultural Research Corporation (EMBRAPA Goats and Sheep), Sobral, Ceará (CE), Brazil. The site is located in a semiarid region that is 70m above the sea level with a prevailing slope ranging from three to 20%. The mean annual temperature and rainfall are 27°C and 822mm, respectively, with rainfall mainly concentrated from February to May.20 The climatological data for the study period are shown in (Table 1). The area soils are typical Orphic Chromic and Hypo chromic Luvisols.21 The predominant vegetation is medium Caatinga forest, which contains deciduous, thorny, trees and scattered evergreen trees,22 although a new independent unit may occur in Sobral and other Ceará regions because of the dominance of Cordia oncocalyx Allemão (common name: pau-branco).23
Parameters |
Rainy Months² |
August |
September |
October |
Total Rainfall (mm) |
845.6 |
0.0 |
0.0 |
0.0 |
Mean Tair (ºC) |
25.7±3.86 |
27.4±6,05 |
28.7±6,59 |
28.9±5,75 |
RAH (%) |
76.2±20,5 |
60.9±22.69 |
53.4±23.04 |
56.1±21.18 |
Tsoil 5cm (°C) |
27.3±2.99 |
30.1±3,17 |
32.9±3.51 |
34.3±2.82 |
VSM 30cm (m3 m-3) |
0.39±0.0078 |
0.25±0.0099 |
0.24±0.0026 |
0.23±0.0047 |
VSM 50 cm (m3 m-3) |
0.33±0.0014 |
0.19±0.0001 |
0.18±0.0011 |
0.17±0.0009 |
Table 1 Climatological data (± standard deviation) recorded in an agrosilvopastoral system,¹ Crioula Farm, Sobral, Ceará, Brazil
¹Data recorded at a station installed in the study area in 2011. ²Means for January to July (except for rainfall). Tair, air temperature; RAH, relative air humidity; Tsoil, soil temperature; VSM, volumetric soil moisture
A long-term experiment has been conducted since 1997 to assess agroforestry alternatives to traditional and conventional agricultural systems in the region. The systems assessed in the experiment conducted by Embrapa Goats and Sheep include traditional cultivation systems, agrosilvopastoral and silvopastoral systems, and native reserve areas used as controls. The agrosilvopastoral system (ASP) was selected for this study. It encompasses an area of 1.6 ha, and alley cropping with 3.0-m wide tracks was adopted with maize (Zea mays L.) and/or sorghum (Sorghum bicolor L. Moench) interspersed with leucaena rows [Leucaena leucocephala (Lam.) de Wit] implanted with 0.5m spacing between plants. This system consists of approximately 200 trees per ha, corresponding to 22% groundcover. No irrigation or fertilisers are applied, and all management is manual. The organic material derived from leaves and branches cut at the beginning of the experiment has been incorporated into the soil. Additionally, the input of organic matter and minerals occurs continuously through the decomposition of falling leaves and branches, biomass derived from the pruning of leucaena and native trees conducted at the beginning of the rainy season and manure from grazing animals (sheep and goats) after the crop harvest.
Species assessed and experimental design
The specie Cordia oncocalyx Allemão (Boraginaceae) was selected because of its high frequency (50% in the ASP).24 C. oncocalyx trees are deciduous, reach up to 12m high, produce leaves and bloom early in the rainy season (January/February). Fructification starts in March, and the fruits persist until the beginning of the dry season (July/August) when the plants lose their leaves. Trees in the ASP have a single trunk with mean diameter at breast height (DBH) of 30cm. The abundance of C. oncocalyx is 80 specimens per ha in the ASP.
For the analysis of leaf nutrients, sun leaves were collected from February to September and shade leaves were collected in July and August 2011 from five flowerless and fruitless trees selected for their similarity in diameter at breast height (DBH) and height. The harvested leaves were fully expanded and mature, with no apparent marks of predation or parasitism.25 The mean values of sun leaves from February to July were clustered and represent the results of the rainy season. The dry season is represented by data from August (30 days after the last rain-dar) and September (50dar). Regarding the shade leaves, samples from July represent the end of the rainy season and samples from August represent the dry season.
Effect of trees on the cultivation
Maize (Zea mays L. var. catingueiro) was the species assessed in the agrosilvopastoral system (ASP). Five 8mx8m plots were outlined to assess the effect of trees on cultivation, with a specimen of pau-branco in the centre and four north-south rows of maize growing 1 m apart on both sides of C. oncocalyx. There were 64 plants in the plot and 16 plants per treatment. The maize was sown in February and harvested in May 2011, following a 90-day life cycle.
Maize was harvested at four different distances from the trunk of C. oncocalx to assess the effect of trees on the maize nutrient concentrations: 1.0m (Mai1), 2.0m (Mai2), 3.0m (Mai3) and 4.0m (Mai4). Considering the canopy projection of C. oncocalyx at noon, the maize plants were located under the canopy at the first two distances (Mai1 and Mai2), whereas they were on the edge of the canopy projection at the third distance (Mai3) and fully exposed to the sun (outside the canopy) in the fourth (Mai4).
All of the fully expanded leaves were used to obtain sufficient material for the analysis of maize leaf nutrients, and the leaf sheaths were removed. One row of plants containing the four treatments was collected per plot in the east-west direction. Only material collected 60 days after sowing (DAS) was in adequate condition for analysis because the material collected 30DAS was insufficient and leaves collected 90DAS were already in senescence.
Soil chemical analysis
Samples were collected at depths of 0-10, 10-20 and 20–40cm and at 0.20m (ASPunder) and 4.0m (ASPoutside) from the trunk of pau-branco, for the soil chemical assessments. All of the sample collections were performed in February at the start of the rainy season, before growing the maize. In total, 24 soil samples were collected, corresponding to four samples per depth at two distances from the trunk.
The samples were air dried sieved (2.0mm mesh) and stored. The concentrations of SOC, TN, exchangeable bases (Na+, Ca2+ and Mg2+), available P and K, pH in water (1:2.5) and micronutrients (Fe, Zn, Cu and Mn) were assessed. The analyses were performed according to,26 except for SOC, which was assessed by the method described by,27 and includes oxidation with potassium dichromate solution and titration with ferrous ammonium sulphate, and TN, which was assessed according to,28 by digestion at 350°C in H2SO4, K2SO4 and CuSO4 catalyst mixture and subsequent distillation and titration. Mehlich-1 was used as an extractor for the assessment of available P and K and exchangeable Na+ and K+, and ammonium acetate at pH 7.0 and titration with ethylenediaminetetraacetic acid (EDTA) were used for the exchangeable Ca2+ and Mg2+. Available Na+ and K were assessed by flame photometry and available P was assessed by colourimetry. Mehlich-1 was used as an extractor for the assessment of micronutrients at a 1:10 (soil:extractor) ratio, and the concentrations of micronutrients were assessed by atomic absorption spectrophotometry.
Analyses of leaf nutrients
The pau-branco and maize leaf samples were dried in an oven at 65ºC until reaching a constant weight and then ground, sieved (1.0-mm mesh) and stored for the analysis of macronutrients (N, P, K, Ca and Mg) and micronutrients (Fe, Zn, Cu and Mn). The procedure used in the assessment of leaf N was the semi-micro-Kjeldahl method.28 For the other nutrients, wet digestion was performed using a mixture of nitric and perchloric acids at a 3:1 ratio according to the method proposed by,29 P was assessed by colourimetry; K was assessed by flame photometry; and Ca, Mg and micronutrients were assessed by atomic absorption spectrophotometry.
Calculation of nutrient inputs and outputs
The contribution of nutrient concentrations by pau-branco was weighted according to the total dry mass (TDM) production per tree multiplied by the mean concentrations of each nutrient in sun and shade leaves separately. The total per tree (TPT) was obtained by adding the sun and shade leaves, and the total concentration per system (TPS) were obtained by multiplying the TPT by the total number of pau-branco trees from 1.0ha of the agrosilvopastoral system. The TDM values were obtained from,30 The estimated maize nutrient export was weighted after calculating the percentage of each nutrient in relation to the dry mass production of shoots, which was recorded by Mendes MM et al,31; this amount was then multiplied by the number of plants in each distance in the plot. The total per plot (TPP) was obtained by the sum of estimates per treatment (Mai1-Mai4), and the total per ha (TPH) was weighted considering the value of each plot (TPP) per 1.0ha.
Statistical analyses
An analysis of variance (ANOVA) followed by Tukey’s test, at 5% significance was used to assess the significant differences between the nutrient concentrations of pau-branco sun leaves in the periods studied (rainy months and 30dar and 50dar) and between the means of the leaf nutrients and estimates of maize nutrients exported in the four ASP treatments (Mai1, Mai2, Mai3 and Mai4). Student’s t-test was used to assess differences between the means of soil chemical variables (ASPunder and ASPoutside) as well as the nutrient concentrations of pau-branco shade leaves. The plots were designed using the software Microcal OriginTM.32
The soil concentrations of TN, Ca2+ and available P, Fe, Zn and Mn in the 0-10cm layer were higher in the ASP under the C. oncalix canopy (ASPunder) (Table 2), although this observation may have resulted from the effect of the canopy and trunk intercepting more than 10% of the available rainwater depending on the crown type and trunk diameter.33 Thus, the physical impact of raindrops was attenuated, and the stability of aggregates was maintained, which reduced the amount of transported materials and the soil nutrient losses under the canopy, especially of minerals with low mobility in the soil, including P, whose primary loss process is leaching.34 Furthermore, the increased nutrient concentrations may have resulted from the decomposition of leaves accumulated under the canopy, exudation from roots or from stemflow, which is the transport of leaf and trunk nutrients, especially K+, by rainwater,1, 35, 36 therefore, the nutrients remain concentrated near the trunk base.2
System |
pH |
TN |
SOC |
Ca2+ |
Mg2+ |
Na+ |
K+ |
P |
Fe |
Cu |
Zn |
Mn |
|
g kg-1 |
cmolc dm-3 |
mg kg-1 |
|||||||||
|
0-10 cm depth |
|
||||||||||
ASPunder |
6.76a |
2.41a |
33.42a |
8.18a |
2.05a |
28.00a |
269.50a |
102.04a |
45.63a |
0.69a |
6.86a |
178.53a |
ASPoutside |
6.81a |
1.22b |
24.25a |
6.78a |
2.55a |
21.00a |
186.00b |
33.23b |
14.28b |
0.33b |
2.27b |
80.96b |
|
10-20 cm depth |
|
||||||||||
ASPunder |
6.39a |
0.59a |
27.38a |
5.57b |
2.65a |
29.50a |
121.00a |
41.21a |
20.57a |
0.36a |
2.30a |
66.34ª |
ASPoutside |
6.36a |
0.42a |
20.77a |
8.70a |
3.85a |
33.50a |
84.50b |
6.20b |
27.01a |
0.54a |
1.37b |
57.75ª |
|
20-40 cm depth |
|
||||||||||
ASPunder |
5.98a |
0.52a |
12.61a |
6.75a |
3.55a |
44.50a |
72.00a |
24.35a |
37.68a |
0.72a |
1.51ª |
59.00a |
ASPoutside |
6.33a |
0.35b |
21.69a |
6.17a |
3.92a |
32.50a |
57.50a |
2.33b |
19.43b |
0.81a |
1.70ª |
49.44ª |
Table 2 Chemical properties of a Luvisol under agrosilvopastoral (ASP) management at 0.20m (ASPunder) and 4.0m (ASPoutside) away from the trunk of Cordia oncocalyx, Crioula Farm, Sobral, Ceará, Brazil
Values between treatments with different lowercase letters and among depths with different uppercase letters differ according to Student’s t test (p<0.05), n=4
Cordia oncocalyx trees showed differences in the nutrient concentrations of sun leaves between the rainy months (RM) and 50 dar (Figure 1). At 50 dar, the air temperature is higher, no rainfall occurs and air humidity and soil moisture are lower (Table 1). Under these conditions, plants begin to physiologically prepare for the dry season. This preparation involves nutrient redistribution and loss of leaves (leaf shedding).
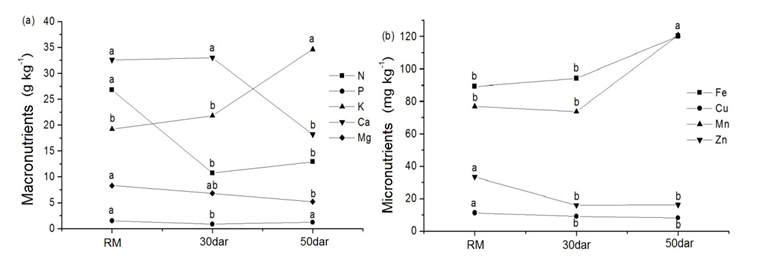
Figure 1 Concentrations of macronutrients (a) and micronutrients (b) in the sun leaves of Cordia oncocalyx in an agrosilvopastoral system in the rainy season (RM), 30days after the last rain (dar) and 50dar. Different letters indicate significant differences between seasons according to Tukey’s test (p<0.05), n=5.
Thus, the macronutrients N, Ca and Mg tended to decrease between the rainy and dry seasons, whereas K increased and P varied little between seasons. The micronutrients Fe and Mn tended to increase, and Cu and Zn tended to decrease as the dry season progressed. In the phloem, N, P, K and Mg are mobile elements, whereas Cu, Zn, Mn and Fe are not particularly mobile, and Ca is immobile. Therefore, the concentrations of nutrients with high or low mobility would be expected to decrease in the dry season because of the relocation of these nutrients from source organs (senescent leaves) to sinks (younger leaves), which did occur for some nutrients (N, Mg, Cu and Zn). However, K, Fe and Mn displayed an atypical pattern and were found at high concentrations in the dry season, indicating that for these elements, the leaves still remain physiologically active and function as sources. Accordingly, the high concentrations of these nutrients may help to maintain the integrity of chloroplasts and photosynthesis in the dry season,37 which was observed for C. oncaclyx in an agrosilvopastoral system.30
The higher values of all nutrients recorded in the trees compared to the soil indicate that a large proportion of minerals is stored in plants, which is observed in tropical forests and not temperate forests, where mineral nutrients are stored predominantly in litter fall, which decomposes in the soil for extended periods of time.1 The concentrations of certain macronutrients or micronutrients in C. oncocalyx shade leaves were lower than in the sun leaves. The mean values of N and Zn recorded in the rainy season in shade leaves were lower than half the values recorded for sun leaves, whereas the K concentrations could be 10 times lower in shade leaves (Figure 1, Table 3). The concentrations of P, Ca, Mg, Fe, Cu and Mn were similar in both leaf types. Leaves from branches inside the canopy, which grow under conditions of low solar radiation interception, typically have larger specific leaf area and lower photosynthetic capacity.38 These characteristics explain why such leaves had lower content or concentrations of mineral nutrients.
Mineral nutrients |
Rainy season |
30 dar |
Mineral nutrients |
Rainy season |
30 dar |
Macronutrients (g kg-1) |
Micronutrients (mg kg-1) |
||||
N |
12.10a |
9.74b |
Fe |
96.17a |
82.82b |
P |
1.03a |
0.88b |
Cu |
16.82a |
7.04b |
K |
2.56a |
2.88a |
Zn |
16.70a |
12.82b |
Ca |
34.11b |
41.01a |
Mn |
72.39a |
81.05a |
Mg |
8.51a |
7.40b |
|
|
|
Table 3 Concentrations of macronutrients and micronutrients in shade leaves of Cordia oncocalyx in an agrosilvopastoral system at the end of the rainy season and 30 days after the last rain (dar)
Means followed by different letters are significantly different between seasons according to Student’s t test (p<0.05), n=5
The concentrations of a significant number of nutrients (N, P, Mg, Fe, Cu and Zn) in shade leaves decreased between the rainy and dry seasons (Table 3), characterising the redistribution of these nutrients upon temporal proximity to leaf senescence. Only K and Mn remained constant between seasons, whereas the concentration of Ca increased by 7.0g between the dry and rainy seasons, indicating that the leaves were still physiologically active and working as sinks.
The concentrations of N, K, Mg, Fe, Cu and Zn in the leaves of maize grown in the ASP did not differ (Table 4), indicating an absence of tree effects on the uptake and translocation of these nutrients, although the trees had an effect on the gas exchange of maize plants,31,39 which affects nutrient absorption. However, P showed a tendency to increase and Ca and Mn showed a tendency to decrease with distance from the trunk of C. oncocalyx. Ca and Mn are mineral elements that are considered practically immobile in the phloem; thus, following their absorption and transport via the xylem, they reach the leaves and remain there without being translocated or distributed.37 Because maize plants grow less under the effect of tree shading,31 these “imprisoned” elements may be found at higher concentrations in plant leaves under the canopy. P is a highly element mobile in the phloem, and it may be redistributed from sources (mature leaves) to sinks (inflorescences, fruits and roots, for example) and be found at lower concentrations in leaves.
Treatment |
Macronutrients (g kg-1) |
|
|
Micronutrients (mg kg-1) |
|
||||
N |
P |
K |
Ca |
Mg |
Fe |
Cu |
Zn |
Mn |
|
Mai1 |
11.51a |
0.78a |
28.48a |
1.82a |
2.95a |
695.99a |
10.37a |
67.46a |
44.49a |
Mai2 |
11.59a |
1.42ab |
27.52a |
1.09b |
3.17a |
538.44b |
8.56b |
73.07a |
40.83a |
Mai3 |
12.39a |
2.44b |
29.28a |
1.34ab |
3.40a |
600.44a |
9.59ab |
77.04a |
36.16b |
Mai4 |
11.52a |
2.16b |
26.72a |
0.30c |
3.07a |
630.22a |
9.23ab |
73.68a |
35.60b |
Table 4 Concentrations of macronutrients and micronutrients in leaves of maize grown in an agrosilvopastoral system 1 m (Mai1), 2 m (Mai2), 3 m (Mai3) and 4 m (Mai4) from the trunk of Cordia oncocalyx
Data in the same column with different lowercase letters are significantly different according to Tukey’s test (p<0.05), n=5
Nutrient input through pau-branco litter decomposition may surpass 400g Ca, 200g N and nearly 200g K per tree (Table 5) depending on the total pau-branco leaf fall in the dry season. Considering the number of trees in the ASP, these trees may contribute up to 35kg Ca, nearly 19 kg N and slightly over 15kg K and 1.0Kg P over 1.0ha. The contribution of Ca may help maintain a soil pH suitable for plant growth and eliminate the need to perform liming. A study conducted by,40 showed that leaves from Caatinga plants usually have at least 10 to 15 g kg-1 Ca; however, the means ranged from 18 to 43 g kg-1 in C. oncocalyx, where were values well above those previously recorded.
Mineral nutrients |
Sun leaves (g)* |
Shade leaves (g)* |
TPT (g tree-1) |
TPS |
N |
204.49 |
32.91 |
237.41 |
18.99 |
P |
12.76 |
2.80 |
15.56 |
1.24 |
K |
185.62 |
6.96 |
192.58 |
15.40 |
Ca |
353.73 |
92.78 |
446.51 |
35.72 |
MG |
90.59 |
23.15 |
113.74 |
9.09 |
Fe |
1.43 |
0.260 |
1.69 |
0.135 |
Cu |
0.18 |
0.045 |
0.235 |
0.019 |
Zn |
0.30 |
0.045 |
0.351 |
0.028 |
Mn |
0.58 |
0.195 |
0.776 |
0.062 |
Table 5 Contribution of sun and shade leaves to the nutrient concentrations per tree (TPT) and nutrient input into the agrosilvopastoral system (TPS)
* Calculation performed by considering the mean dry weight of sun and shade leaves per tree in the system
Maize plants grown 4m from the trunk of C. oncocalyx (Mai4) showed the highest concentrations of all nutrients (Table 6), and this result is associated with their greater photosynthetic capacity and development compared to that of the shaded plants.31 The exception was Ca, which showed the highest concentrations in plants 2.0 and 3.0m from the trunk (Table 6). The plants at Mai4 also showed the greatest increase in concentrations of nutrients exported with the shoot harvest, either through direct grazing of goats and sheep, for silage production or even for feeding the goats in the trough. Approximately 35g K and 15g N per plot (equivalent to 0.064ha) may be exported from the system and this value increases substantially when considering the entire 1.0 ha area; therefore, more than 5.0kg K and 2.0kg N may leave the system (Table 6). Because nutrient absorption increases in the grain-filling stage,41 and grains are also removed in the harvest, these numbers can increase significantly and represent an even greater loss of nutrients from the system. However, because the goats access the area after the harvest, a portion of the nutrients is returned as faeces or manure.
Treatments |
Macronutrients (g) |
Micronutrients (g) |
|||||||
N |
P |
K |
Ca |
Mg |
Fe |
Cu |
Zn |
Mn |
|
Mai1 |
1.85c |
0.125c |
4.58c |
0.293b |
0.474c |
0.112b |
0.0016c |
0.0108c |
0.0071c |
Mai2 |
4.12b |
0.505b |
9.79b |
0.388a |
1.127b |
0.191b |
0.0031b |
0.0259b |
0.0146b |
Mai3 |
3.64b |
0.717b |
8.61b |
0.394a |
0.999b |
0.176b |
0.0028b |
0.0226b |
0.0106bc |
Mai4 |
5.46a |
1.023a |
12.66a |
0.142c |
1.455a |
0.299a |
0.0042a |
0.0351a |
0.0171a |
TPP (g) |
15.08 |
2.371 |
35.64 |
1.217 |
4.057 |
0.778 |
0.0117 |
0.0944 |
0.0493 |
TPH (g) |
2355.65 |
370.53 |
5568.99 |
190.10 |
633.84 |
121.58 |
1.829 |
14.758 |
7.704 |
Table 6 Export of leaf macronutrients and micronutrients with the removal of maize plants grown 1 m (Mai1), 2m (Mai2), 3m (Mai3) and 4 m (Mai4) from the trunk of Cordia oncocalyx in an agro forestry system
Data in the same column with different lowercase letters are significantly different according to Tukey’s test (p<0.05), n=5, TPP, total per plot; TPH, total per ha
The presence of trees in the agrosilvopastoral system contributes to maintaining the soil chemical quality, which is shown by the higher concentrations of nutrients under the Cordia oncocalyx canopy. Although these higher soil concentrations have little effect on the nutrient concentrations of maize plants, the amount of nutrients that returns to the system with the fall of C. oncocalyx leaves is sufficient to replace the losses from partly removing the maize. Thus, the presence of trees becomes critical to nutrient cycling and maintenance of soil fertility, and it can eliminate the need for external inputs for the development of agricultural practices.
None.
We would like to thank Empresa Brasileira de Pesquisa Agropecuária (Embrapa Goats and Sheep) for allowing the field work for this study. We are also grateful to the Coordenadoria de Aperfeiçoamento de Pessoal de Ensino Superior - CAPES and the Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq for the study and research-productivity grants awarded to the authors and for the resources allocated towards development of the research.
Authors declare that there is no conflict of interest.

©2019 MMDS,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.