eISSN: 2577-8307


Research Article Volume 4 Issue 1
1Holetta Agricultural Research Center, Ethiopia
2Central Ethiopia Environment and Forest Research Centre, Ethiopia
3Environmental Science Centre Programme, College of Natural Science, Ethiopia
Correspondence: Mehari Alebachew Tesfaye, Central Ethiopia Environment and Forest Research Centre box 30708, Addis Ababa, Ethiopia
Received: January 10, 2020 | Published: February 27, 2020
Citation: Amha Y, Tesfaye MA, Kassa Z, et al. Growth and biomass production of some selected native and introduced tree/shrub species under severely degraded landscapes of west showa zone of oromiya regional state, central highlands of Ethiopia. Forest Res Eng Int J. 2020;4(1):28-34. DOI: 10.15406/freij.2020.04.00096
Rehabilitation of degraded lands through tree/shrub planting could improve land productivity, rural livelihoods, biodiversity and other ecological services. The growth and early performance of six exotic, five indigenous, and a mixture of both origins were evaluated on a severely degraded land of Fotololo, West Shoa, Ethiopia. A total of sixteen treatments were arranged in randomized complete block design with three replications. Each plot contained 25 trees/shrub species planted at the spacing of 1.5m x 1.5m. The survival percentage of indigenous species after 42 months was generally lower than the exotic ones. The lowest survival percentage among indigenous species was recorded by Albizia gummifera (J.F.Gmel.) C.A.Sm. (4%) while the highest was recorded by Olea europaea subsp. cuspidata (Wall. & G.Don) Cif. (51%). Among the exotic species, Sesbania sesban (L.) Merr. and Acacia saligna (Labill.) Wendl. had the highest survival percentage (80 and 71%, respectively) but Acacia decurrens Willd. had the lowest rate (20%). The maximum height 3.75m and root collar diameter 49.9mm were attained by S. sesban (L.) Merr. followed by Acacia saligna (Labill.) Wendl. and Eucalyptus camaldulensis Dehnh. However, all indigenous species had grown poorly, with the height growth range of 0.52±0.39m (Albizia gummifera (J.F.Gmel.) C.A.Sm.) to 1.20±0.26m (Millettia ferruginea (Hochst.)). The growth and survival of C. africana Lam., Juniperus procera Hochst. ex Endl., Millettia ferruginea (Hochst.) and Olea europaea subsp. cuspidata (Wall. & G.Don) Cif. were slightly improved when they were planted in mixture with either of the following exotic species. The dry biomass of the undergrowth species varied between 448 gm-2 (from Olea europaea subsp. cuspidata (Wall. & G.Don) Cif. plot) and 952 gm-2 (from Sesbania sesban (L.) Merr. plot. Rhodes was the dominant species in the experimental site and followed by Digitaria, Cyanadon and Trifolium species. In conclusion Sesbania sesban (L.) Merr. and Acacia saligna (Labill.) Wendl. are the outstanding species with best growths and survival rate and they can be used as entry species to rehabilitate degraded lands at Fotololo and other areas with similar agro ecological conditions.
Keywords: degraded lands, diameter, exotic species, height, indigenous species, survival
Land degradation is one of the main environmental problems in the central highlands of Ethiopia.1 The Ethiopian highlands cover 44% of the nation’s total landmass, and are generally characterized by the presence of high human and livestock population. Natural forests in these areas have so long been giving enormous benefits to millions of its dwellers as source of woody materials, nutrition, health care and cash income. However, such forest lands have dwindled in the last millennia,2 as a result of high demand for forest products and crop lands. Moreover, declines in soil fertility,3 together with the prevailing climate changes (such as alteration of spatial and temporal patterns in temperature, rainfall, solar insolation and wind) have contributed much for the continuation of deforestation and land degradation in these areas. Hence, restoring degraded lands is a necessity to enhance sustainable rural livelihoods, economic development, conservation of biodiversity, and resolving conflicts over resources.2
Restoration of such degraded lands can be achieved through various means including the use of tree/shrub covers and area closures. Each of these methods should, however, generally help in recovering the productive potential of the sites,4 and meets the economic, social and environmental requirements of the local community. Apart from the provision of woody and non-woody forest products, planting tree/shrub species in degraded sites can potentially improve soil quality through numerous processes including maintenance or increase of soil organic matter (SOM), biological N2 fixation, recycling of leached nutrients beyond the reach of crop roots, increased water infiltration and storage capacity, reduced loss of nutrients by erosion, adjusting soil acidity, and improving soil physical and biological properties.5 To this end, various institutions have been involved in tree and shrub planting schemes to restore degraded sites in the central highlands of Ethiopia. However, the performance of both indigenous and exotic tree/shrub species in these areas was not satisfactory due to poor species-site matching, which requires a backstopping with a well designed tree/shrub screening trials. Therefore, bearing in mind the role of trees/shrubs in rehabilitating and restoring degraded lands, an experiment was established to evaluate the early performance of different exotic and indigenous trees and shrubs on severely degraded site at Fotololo.
General description of the study site
The study was conducted at Fotololo site, which is found in the Tokakotaye district, West Shoa zone, Ethiopia. The agroclimatic zone of Tokakotaye is tepid to cool humid plateau (H2-6), and its climatic condition is favorable for the growth of many mid and highland crops. The length of growing season in H2-6 areas varies from 241 days to 300 days. However, the rugged topography, shallow soil depth and poor drainage are some of the major constraints identified in this sub-zone. The diversity of trees and shrubs in the area is relatively low. The dominant tree around homesteads is Eucalyptus camaldulensis Dehnh., Acacia abyssinica Benth., Olea europaea subsp. cuspidata (Wall. & G.Don) Cif. and other indigenous species are also sparsely grown on riverbanks, farm- and grazing-lands. Fotololo has a rugged and stony terrain with very thin layer of soil cover, which makes it less ideal for plantation development. In former times (especially during Derg regime), efforts have been made to rehabilitate the site with Eucalyptus camaldulensis Dehnh. and Cupressus lusitanica Mill. although these efforts were remained largely unsuccessful (Personal communication with Bureau of Agriculture and Rural Development, 2010, Tokakutaye district).
Site selection procedure and seedling production
A discussion forum was organized with the Tokakotaya wereda office of agriculture and rural development to identify representative site for the project. Based on the severity of land degradation, Fotololo site was chosen. A base line data was then taken using GPS and digital cameras. Geographically, the site is located at 9°11′N and 38°20′E with an elevation of 2100m.asl. The local people abandoned the site before a decade as the site could not be used for the production of crop and livestock. Major actors in this specific site were then identified and responsibilities were shared.
Seeds of most of tree and shrub species planted were obtained from the Forestry Research Centre. However, seeds of Acacia decurrens Willd. were collected from the local mother trees around Holetta Agricultural Research Canter. Some of the seeds were pre-treated according to their needs; and sown directly on a polythene tube that contained a mixture of 4 (local soil): 2 (forest soil): 1 (sand). The forest soil was obtained from the pine plantation. Pine forest soil was used to enrich the soil with organic matter as well as to develop mycorrhizal association with planted species (if needed). The size of the polythene tube was 15 cm (height) by 8 cm (diameter). Seedlings were raised at Guder nursery site with similar nursery management practices (watering, shading, weeding and hardening practices).
Experimental design
This experiment was laid out in randomized complete block design (RCBD) with three replications. The list of tree/shrub species included in the early performance evaluation study is given in Table 1. Each plot had the total size of 56.25 m2, which contained 25 trees/shrub species at spacing of 1.5m x 1.5m. Each planting hole had a depth of 50 cm. Since most of the top soil in the study site is washed away by erosion, local soil (brought from Guder town) was used during refilling. This would provide condition for better root growth. Mulching and watering were carried out during the dry season of the first year. Watering was done for four months (mid-January to mid May) at 7 days interval. There was the prevalence of termite attack during the dry season. Data on survival, height and root collar diameter were taken until week 42 (five times). The grass biomass under each tree/shrub canopy was assessed using a quadrat method. The quadrat had a 50 cm by 50 cm size. Sampling was done at three places by throwing the quadrat randomly inside the plot. This sampling method is often statistically valid and provides crucial information about the types and amount of undergrowth vegetation. The site was fenced with barbed wire to minimize human and livestock interferences.
Species |
Time after planting (month) |
|||
6 |
12 |
18 |
30 |
|
1. Acacia decurrens Willd. |
47±6 |
35±6 |
23±7 |
20±2 |
2. Acacia saligna (Labill.) Wendl. |
85±7 |
77±7 |
76±0 |
71±14 |
3. Casuarina equisetifolia L. |
60±0 |
49±7 |
41±3 |
41±13 |
4. G. robusta A.Cunn. ex R.Br. |
74±4 |
51±9 |
41±10 |
41±11 |
5. E. camaldulensis Dehnh.. |
75±4 |
57±1 |
56±5 |
56±5 |
6. Sesbania sesban (L.) Merr. |
87±6 |
87±8 |
87±2 |
80±9 |
7. Millettia ferruginea (Hochst.) |
69±7 |
33±5 |
8±0 |
4±4 |
8. C. africana Lam. |
77±13 |
49±7 |
17±3 |
12±5 |
9. M. ferruginea (Hochst.) |
87±8 |
63±5 |
59±1 |
44±12 |
10. J. procera Hochst. ex Endl. |
53±15 |
51±8 |
46±0 |
42±9 |
11. O. europaea subsp. cuspidata (Wall. & G.Don) Cif. |
64±10 |
59±3 |
56±0 |
51±1 |
12. Acacia decurrens Willd.+C. equisetifolia L. |
67±7 |
43±5 |
43±0 |
38±9 |
13. Sesbania sesban (L.) Merr.+O. europaea subsp. cuspidata (Wall. & G.Don) Cif. |
68±2 |
47±8 |
43±3 |
47±2 |
14. E. camaldulensis Dehnh. .+J. procera Hochst. ex Endl. |
52±8 |
41±3 |
41±5 |
37±11 |
15. Casuarina equisetifolia L.+M. ferruginea (Hochst.) |
77±5 |
56±16 |
39±1 |
39±9 |
16. Sesbania sesban (L.) Merr.+C. africana Lam. |
85±3 |
61±5 |
48±4 |
35±7 |
Mean±SEa |
71±4 |
56±5 |
46±7 |
42±7 |
Table 1 Mean survival percentage of tree/shrub species (%) planted at Fotololo
aOveral mean excluding mixed species±standard error
Soils in the study site were predominantly red in color. Initial soil sample was collected at a depth of 0 - 10 cm, 10 - 30 cm, and 30 - 50 cm; and used as a baseline data to evaluate changes in soil fertility parameters over the project span, which was five years. Soil parameters such as pH (1:2.5 water), total N (%), organic C (%), available P (mg kg-1 dry soil), available K (mg kg-1 dry soil) and CEC (meq/100g dry soil) were measured using appropriate soil laboratory procedures.
Initial soil characteristics
Improper farming system, undulated topography, and high rainfall at Fotololo have led subsequently to severe erosion and exposed nutrient-poor and unproductive sodic subsoil (Table 2), as indicated by high pH, low OM content and poor N and P contents. The pH values observed in the experimental sites ranged from 7.99 to 8.15, and showed a slightly increasing trend along the soil depths. SOM is very important in fertility management as it improves soil fertility and structure. Total organic C, which accounted nearly 58% of SOM, tended to decrease with depth (Table 2). Fertile top soils have an average OM content of 5%,6 showing poor fertility status in the current experimental site (only 1.6% for the top 0 - 10 cm depth). This is because soils with comparatively higher OM content are considered more fertile than soils with low OM content. The total N content in the 0-10, 10-30, and 30-50cmwas 0.06, 0.05 and 0.03%, respectively (Table 2), and according to Barbar,7 the soil was generally characterized as very low in N content. Total nitrogen is, however, merely an indicator of the soil potential for the element as it does not fully become available to the plant. According to Buruah and Barthakur,8 nearly 95 – 99% of total N is in the organic form and only 1 – 5% is found in the form of inorganic N (ammonium and nitrates). The C/N ratio of this soil remained constant along the three depths (C/N=15).
Soil depth (cm) |
pH (1:2.5 water) |
Total N (%) |
Organic Carbon (%) |
P Olson (mg kg-1 dry soil) |
K (mg kg-1 dry soil) |
CEC (meq/100g dry soil) |
0-10 |
7.99±0.20a |
0.06±0.01 |
0.91±0.10 |
1.67±0.12 |
0.89±0.14 |
20.26±4.29 |
30-Oct |
8.05±0.10 |
0.05±0.01 |
0.75±0.14 |
1.60±0.20 |
0.90±0.21 |
21.86±5.46 |
30-50 |
8.15±0.09 |
0.03±0.01 |
0.51±0.20 |
1.67±0.23 |
0.84±0.10 |
23.50±0.82 |
Table 2 Some of the initial characteristics of soil at Fotololo site
amean±standard deviation (n=9)
Plants can only take up P in the orthophosphate form; and the available P determined by Olson method ranged from 1.60 to 1.67mgkg-1 dry soil (Table 2). The very low P content of this soil (< 5 mg kg -1 dry soil7) can partly be explained by its low level of SOM as SOM is the main source of orthophosphate in most soils.8 Available K in the soil extract ranged from 8.4 to 9.0 mg kg-1 dry soil, with no trend along depth. Available K in the soil is generally influenced by the amount and type of clay mineral, cation exchange capacity (CEC), K buffering capacity and soil pH. The cation exchange capacity increased with increasing soil depths (Table 2). In general, tree planting in degraded lands could immensely improve soil physical (e.g., bulk density, water holding capacity), chemical (e.g., nutritional status, soil pH), and biological properties (e.g., microbial activity and their composition). However, the measured soil characteristics in the experimental site suggest that they may adversely affect the survival and growth of both indigenous and exotic tree and shrub species.
Survival percentage
Since planting of both indigenous and exotic species can play major roles in restoring the productivity, ecosystem stability, and biological diversity of degraded lands,9 a total of sixteen treatments (six exotic and five indigenous trees and shrubs, and a mixture of both) were evaluated for their early performance on a severely degraded land at Fotololo, west Shewa, Ethiopia. The survival percentage showed greater difference between exotic and indigenous species; and the highest mortality was mostly pertained by the later group (Table 1). The overall survival percentage of six exotic and five indigenous tree/shrub species after 6, 12, 18 and 30 cm depth was 71, 56, 46 and 42%, respectively.
The survival rates of all tree/shrub species decreased considerably over time due to extended dry seasons and termite incidences. In order to avoid massive plant death due to desiccation, all species were watered during the first year of their establishment although the practice was withdrawn in subsequent months. Chemicals were also sprayed to reduce the effect of termites on plant growth and survival. Among the tested species, the highest survival rate after 42 months was recorded for S. sesban (L.) Merr. and the lowest survival rate was recorded for Albizia gummifera (J.F.Gmel.) C.A.Sm. Five species namely S. sesban (L.) Merr., A. saligna (Labill.) Wendl. , E. camaldulensis Dehnh., O. europaea subsp. cuspidata (Wall. & G.Don) Cif. and M. ferruginea (Hochst.) showed higher survival percentage after 42 weeks compared to the overall mean survival percentage (Figure 1). However, the altitudinal range of Fotololo is best suited for most of the tested species.10 The observed high mortality rates are, therefore, partly explained by severe termite attack or poor inherent soil characteristics (Table 1). The survival percentage of indigenous species was slightly improved when they were grown in mixture with exotic species (Table 1).
Height and diameter growth
Trees and shrubs (e.g. N-fixing versus non – N-fixing species, coniferous versus deciduous) could play different roles in restoring degraded lands. Nitrogen-fixing species like Sesbania sesban (L.) Merr. and Acacia species can increase soil C and N in the recovery of degraded land in the tropics and subtropics11. Planting A. saligna (Labill.) Wendl. on extremely degraded lands of the country is the most.11 common practice before introducing slow growing indigenous and exotic species that do not tolerate the environmental stresses including water shortage and poor soil conditions. This is because the presence of Acacia species in such degraded land could improve soil quality and facilitate the quick increment of the population of other plant species that provide income plus protection to the area.12 The pattern of height growth over time is shown in Table 3. After 42 months, S. sesban (L.) Merr. attained the maximum height growth, which was followed by Acacia saligna (Labill.) Wendl. and Casuarina equisetifolia L. (Figure 2). In contrast, the lowest height growth was measured from indigenous species in the range of 0.52m Albizia gummifera (J.F.Gmel.) C.A.Sm. to 1.20m (C. africana Lam.). Similarly, both Acacia saligna (Labill.) Wendl. and Sesbania sesban (L.) Merr. attained the highest root collar diameter growth while the lowest root collar diameter growth was attained by Albizia gummifera (J.F.Gmel.) C.A.Sm., J. procera Hochst. ex Endl. and O. europaea subsp. cuspidata (Wall. & G.Don) Cif. (Table 4) (Figure 2) However, the relative growth of most species decreased with time. These results were in line with,13 who reported a higher height and biomass increment at early stage than late ages.
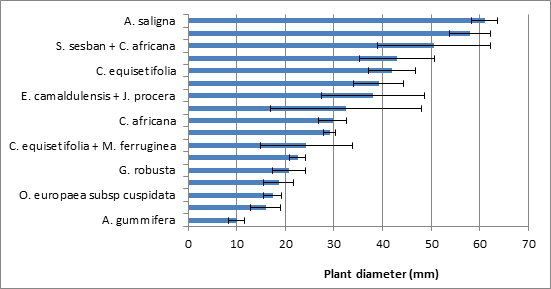
Figure 3 Mean root collar diameter (±standard error) of species and species mixes at the age of 42 months.
Species |
Time after planting (month) |
|||
6 |
12 |
18 |
30 |
|
1. Acacia decurrens Willd. |
0.39±0.04 |
1.20±0.09 |
1.60±0.09 |
2.30±0.05 |
2. Acacia saligna (Labill.) Wendl. |
0.96±0.09 |
2.27±0.03 |
3.08±0.19 |
3.71±0.05 |
3. Casuarina equisetifolia L. |
0.77±0.08 |
1.64±0.19 |
1.88±0.46 |
2.52±0.44 |
4. G. robusta A.Cunn. ex R.Br. |
0.46±0.02 |
0.98±0.13 |
1.26±0.03 |
1.40±0.31 |
5. E. camaldulensis Dehnh. . |
0.73±0.03 |
1.12±0.30 |
1.92±0.35 |
2.44±0.40 |
6. Sesbania sesban (L.) Merr. |
2.46±0.06 |
2.95±0.08 |
3.30±0.24 |
3.75±0.25 |
7. A. gummifera (J.F.Gmel.) C.A.Sm. |
0.18±0.01 |
0.31±0.04 |
0.39±0.06 |
0.41±0.16 |
8. C. africana Lam. |
0.58±0.02 |
0.70±0.24 |
0.76±0.09 |
0.91±0.07 |
9. M. ferruginea (Hochst.) |
0.40±0.02 |
0.70±0.09 |
0.76±0.24 |
0.87±0.14 |
10. J. procera Hochst. ex Endl. |
0.29±0.01 |
0.44±0.06 |
0.54±0.06 |
0.68±0.08 |
11. O. europaea subsp. cuspidata (Wall. & G.Don) Cif. |
0.35±0.03 |
0.53±0.04 |
0.73±0.08 |
0.77±0.06 |
12. Acacia decurrens Willd.+C. equisetifolia L. |
0.53±0.11 |
0.82±0.26 |
1.17±0.23 |
0.94±0.39 |
13. Sesbania sesban (L.) Merr.+O. europaea subsp. cuspidata (Wall. & G.Don) Cif. |
1.72±0.23 |
2.13±0.24 |
2.31±0.01 |
2.79±0.23 |
14. E. camaldulensis Dehnh.+J. procera Hochst. ex Endl. |
0.50±0.03 |
0.99±0.09 |
1.25±0.09 |
1.30±0.09 |
15. C. equisetifolia L.+M. ferruginea (Hochst.) |
0.51±0.04 |
0.92±0.03 |
1.14±0.00 |
1.89±0.40 |
16. Sesbania sesban (L.) Merr.+C. africana Lam. |
1.68±0.10 |
1.99±0.16 |
2.79±0.51 |
2.99±0.36 |
Mean±Sea |
0.69±0.19 |
1.17±0.25 |
1.47±0.30 |
1.78±0.36 |
Table 3 Mean height of tree/shrub species and species mixes (m) planted at Fotololo
aOveral mean excluding mixed species±standard error
The lowest height and root collar diameter growths by J. procera Hochst. ex Endl., O. europaea subsp. cuspidata (Wall. & G.DonCif. and Albizia gummifera (J.F.Gmel.) C.A.Sm. might be associated with the slow growing nature of these species. The height and root collar diameter growth of some of these indigenous tree/shrub species tended to improve when they were planted in mixture than planting alone (Table 3) (Table 4). Therefore, mixing these slow-growing indigenous species with fast growing exotic species can be taken as an alternative to hasten the recovery of degraded lands. In this regard, there is increasing evidences on the merits of mixed-species plantations in rehabilitating degraded lands than the single-species plantations,14 by increasing land cover, producing better biomass, attracting animal seed dispersers as well as improving soil fertility. However, replacements of such short-lived shrub species should also be considered at some stage.
Undergrowth biomass
The amount and type of understory vegetation (herbs and grasses) may partly provide valuable information about the degree of land reclamation in a given degraded land. It is because they are frequently cited as most useful plants in combating topsoil erosion as they have shallow, dense and fine root mats. The dry biomass of grass and herbs in a single species plots ranged from 448 (O. europaea subsp. cuspidata (Wall. & G.Don) Cif.) to 952 gm-2 (S. sesban (L.) Merr. with a mean value of 698gm-2 (Table 4). The common grown broadleaved species as undergrowth in the area were Trifolium, Bidens (e.g., Peristinaria), Medicago polimorpha L., Guizotia scabra (Vis.) Chiov. and Scorpurus muricatus whereas the dominant grass species were Digitaria abyssinica, Cyanadon dactylon, Setaria fumila, and Rhodes species. The relative abundance of these broadleaved and grass species indicated that Rhodes species dominated the site, which was followed by Digitaria, Cyanadon and Trifolium species. The total dry biomass in the mixed plot was mostly higher than those two individual species grown separately (Table 4). The environment created under mixed plots might be better for the growth of undergrowth than individual indigenous tree/shrub species, which in turn give higher soil protection from direct rainfall hit. Accordingly rainfall induced erosion can be reduced a hundredfold by maintaining a dense cover of grasses, or herbaceous vegetation. Very few woody plant species (e.g. Acacia abyssinica Benth.) had started to regenerate on the experimental plots regardless of the screened species to suggest that none of the tested species played a specific fostering role.
S Species |
Time after planting (month) |
Grass biomass (g m-2) |
|||
6 |
12 |
18 |
30 |
||
1. Acacia decurrens Willd. |
4.2±0.2 |
13.8±0.3 |
17.2±1.8 |
20.0±0.6 |
746 |
2. Acacia saligna (Labill.) Wendl. |
11.6±1.1 |
25.8±4.0 |
43.0±2.4 |
48.1±3.6 |
880 |
3. Casuarina equisetifolia L. |
6.4±0.8 |
17.2±1.3 |
22.3±4.6 |
27.5±5.4 |
734 |
4. G. robusta A.Cunn. ex R.Br. |
5.5±0.8 |
16.5±0.4 |
30.5±4.0 |
19.1±2.9 |
795 |
5. E. camaldulensis Dehnh. |
9.1±2.8 |
19.5±0.7 |
33.6±1.8 |
33.5±3.3 |
555 |
6. Sesbania sesban (L.) Merr. |
24.9±0.6 |
29.5±4.8 |
47.0±3.2 |
49.9±2.1 |
952 |
7. A. gummifera (J.F.Gmel.) C.A.Sm. |
3.5±0.3 |
10.1±2.2 |
7.7±2.0 |
4.0±0.0 |
589 |
8. C. africana Lam. |
10.8±0.9 |
18.9±2.0 |
23.9±0.3 |
24.1±3.5 |
595 |
9. M. ferruginea (Hochst.) |
5.9±0.6 |
13.8±0.3 |
17.0±3.2 |
18.5±1.1 |
771 |
10. J. procera Hochst. ex Endl. |
3.7±0.1 |
7.2±2.4 |
10.0±1.2 |
11.7±2.0 |
635 |
11. O. europaea subsp. cuspidata (Wall. & G.Don) Cif. |
3.5±0.2 |
10.8±1.6 |
15.2±1.0 |
12.6±1.1 |
448 |
12. Acacia decurrens Willd.+C. equisetifolia L. |
5.1±0.8 |
13.4±1.4 |
15.4±2.6 |
11.7±2.8 |
796 |
13. Sesbania sesban (L.) Merr.+O. europaea subsp. cuspidata (Wall. & G.Don) Cif. |
16.2±1.1 |
22.7±4.8 |
33.1±1.4 |
35.3±8.7 |
925 |
14. E. camaldulensis Dehnh.+J. procera Hochst. ex Endl. |
6.3±0.7 |
17.5±1.4 |
23.7±1.6 |
36.7±8.7 |
893 |
15. Casuarina equisetifolia L.+M. ferruginea (Hochst.) |
6.6±0.6 |
13.4±16.5 |
17.2±1.3 |
22.4±1.9 |
803 |
16. Sesbania sesban (L.) Merr.+C. africana Lam. |
18.0±1.2 |
27.6±1.8 |
42.1±6.6 |
48.9±6.1 |
821 |
Mean±SEa |
10.1±2.8 |
16.7±2.0 |
24.3±3.9 |
24.4±4.4 |
698±44 |
Table 4 Mean root collar diameter (mm) and grass biomass harvested under planted tree/shrub species at Fotololo
aOveral mean excluding mixed species±standard error
Our results generally revealed that Sesbania sesban (L.) Merr., A. saligna (Labill.) Wendl., E. camaldulensis Dehnh. and M. ferruginea (Hochst.) were the outstanding performers in the area. The higher total harvestable dry biomass of undergrowth from exotic-indigenous species mixes plot compared to indigenous tree species plots indicating that growing indigenous tree species in mixture with short-lived and fast growing exotic tree/shrub species brings better soil cover and protection at early stage of the rehabilitation effort. From the obtained results, it can be concluded that S. sesban (L.) Merr. and A. saligna (Labill.) Wendl. can be used as pioneer species to rehabilitate degraded lands at Fotololo and other areas with similar agro ecological conditions as they grow fast and allow good undergrowth (grass and herbs). However, one has to bear in mind that the objectives of rehabilitation and restoration of severely degraded areas cannot be met with selection of tree/shrub species alone. Different soil conservation measures should, therefore, be coupled with pioneer/promising species so as to protect soils and lands from further degradation.
The authors would like to thank Tekele Hundessa, Balcha Regassa, Mestawot Teshome and Kassahun Bekele for their technical support. This work was financed by the Ethiopian Institute of Agricultural Research (EIAR).
The authors declared that they have no competing interests
This research was covered by Plantation and Agro forestry Research Case Team, Forestry Research Process, Ethiopian Institute of Agricultural Research (EIAR), P. Box 2003, Addis Ababa, Ethiopia.

©2020 Amha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.