eISSN: 2577-8307


Research Article Volume 4 Issue 1
1Bonga Agricultural Research Centre, Ethiopia
2Central Ethiopia Environment and Forest Research, Ethiopia
3Oda Bultum Universities, Ethiopia
Correspondence: Mehari Alebachew Tesfaye, Central Ethiopia Environment and Forest Research Centre box 30708, Addis Ababa, Ethiopia
Received: November 27, 2019 | Published: February 28, 2020
Citation: Wodajo A, Tesfaye MA, Mohammed M. Floristic composition and carbon pools along altitudinal gradient: the case of gara–muktar forest, west hararghe zone, eastern Ethiopia. Forest Res Eng Int J. 2020;4(1):42-50. DOI: 10.15406/freij.2020.04.00098
Forests play vital role in combating climate change through carbon sequestration in the atmosphere and serving as a carbon sink in the form of carbon pool systems of forest ecosystems. The species composition and carbon stock in the different carbon pools and analysis of the influence of the environmental gradients were studied by systematic sampling method collecting data in thirty-six quadrant plots of 20x20m each distributed along transect lines. Diameter at breast ≥5cm and total height measured for each tree in the main plot. Above and below ground biomass was estimated using allometric equation, while the litter carbon was estimated by taking 50% of dry biomass as carbon. Soil sample was collected using auguring method and analyzed following Walkley-Black method, while bulk density was performed using core sampling method. The data was analyzed was performed using one way ANOVA of R software. The carbon stocks in aboveground, belowground, litter biomass and soil organic carbon showed distinct variation along environmental gradients. The aboveground and below ground carbon stock was showed a decreasing trend along with increasing altitude, while soil organic carbon and liter carbon showed increasing trend along with increasing in altitude. The mean total above and below ground carbon stocks were 156.60t C ha-1 and 31.32t C ha-1 respectively whereas, litter carbon and soil organic carbon stocks were 2.72t C ha-1 and 125.86t C ha-1 (up to 30cm depth) respectively. The mean total carbon stock density in all carbon pool of Gara-Mukitar forest was found to be 316.6±67.15t C ha-1 from which 49.5% of carbon was contained in the above ground biomass, 9.9% in below ground biomass, 0.9% in litter carbon and 39.8% was stored in soil organic carbon (0-30cm depth). The analysis of carbon stock variation of different carbon pools along altitude of the forest showed a significant variation, whereas the above and belowground carbon stock variation with slope gradient was also significant except soil organic carbon and liter carbon. It result concluded that Gara-muktar Forest is a reservoir of high carbon, since it has a good capacity to sink carbon from the atmosphere having positive role in reduction of greenhouse gases and in contributing to climate change mitigation. Therefore, to enhance the carbon stock of forest in a sustainable way, it should be supported with proper community based forest management system.
Keywords: altitude, biomass, litter carbon, dry afromontane forest, soil organic carbon
Forests provide an important ecological service, wood and non-wood forest product (NFPs) for the well-being of humans at local, national and global levels such as timber, firewood, NTFPs, grazing land, fodder and recreation. Forests mitigate the global climate change serving as sinks and sources of carbon stock.1 reported that the global forests cover over 4billion ha and contribute around 50% global greenhouse gas mitigation. The tropical forests spread over 13.76million km2 areas worldwide and accounted about 60% of the global forest cover and store an estimated 193-229 Pg of carbon in aboveground biomass and recycling 915Gt of carbon each year, through photosynthesis and net primary productivity.2–5
Ethiopia is endowed with various landscape types resulted in different agro-ecological zones and vegetation types. The vegetation types are diverse, varied from tropical rain forest and cloud forests in the southwest to the desert shrubs in the east and northeast.6 The vegetation is provided diverse wood and non-wood forest products (NWFPs) such as: wild coffee, gum, resin, honey, bees wax, herbal medicines and bamboo. They also provide various ecosystem services such as watershed protection, biodiversity conservation and carbon sequestration. The natural high forests of Ethiopia are mainly found in the highlands where annual rainfall distribution and amount is better and higher human and livestock population are found. Dry afro-montane forests and moist afro-montane forests are the dominant vegetation types found in these areas. However, the former are dominant in the Central, Northern and Western Highlands. They contain very complex vegetation type and species composition and diversity. The climates in these vegetation types are characterized by relatively high humidity, limited and unreliable rain and prolonged dry season of six to eight months per year. Vegetation composition and structure would differ across the elevation gradients, topography and disturbances.7
The national carbon stock of Ethiopia was estimated to be 867TGC by Gibbs et al.8 and 2.76billion tons of carbon by Moges et al.9 The discrepancy between these values is due to the different methods and tools used for the authors and the variability in soil, topography and forest types. Majority of the high forests found in Ethiopia are managed primarily for protection and conservation purpose, while commercial utilization is secondary objective, the forestry administration at the Federal level has classified 58 of the most important high forest with an estimated area of 2million ha as National Forest Priority Areas of the country (NFPAs). However, due to deforestation, over two-third of these high forests are heavily disturbed forests and needs appropriate management intervention. The estimated annual height and diameter growth of these forests are below optimum.10 In addition, the existing woodlands and alpine vegetation are also degraded and converted into bush lands, scrublands and agricultural lands.11,12
The carbon storage in forest can be affected by different environmental factors such as altitude, slope, and aspect Bruun et al.13 Consequently, the microclimate is often linked to soil moisture and distribution of particular plant communities,14 on different slope forms. In addition, patterns of tree species distribution further affects carbon stored in forest ecosystem McEwen et al.15 The altitude is one of the most importance environmental gradients that affect biomass, stem size and stand density and amount of soil organic carbon. This means, a significant effect on climatic factors such as temperature and precipitation Sheikh et al.16 According to Bayat et al.17 slope and aspect has significant relationship with biomass in forest areas due to the interaction between solar radiation and soil properties such as soil moisture and nutrient.
The need for accurate estimates of forest biomass is increasing now a day due to the important of forests in global carbon cycle budgeting and sustainable forest management, along with the assessment of forest structure and condition and forest productivity based on sequential changes in biomass.18 The global forest sector initiate the REDD+projects, which stands for Reducing Emissions from Deforestation and Forest Degradation, and (+) the role of sustainable management of forests, conservation and enhancement of forest carbon stocks in developing countries. To successfully implement mitigating policies and strategies in the REDD+line countries need well-authenticated estimates of forest carbon stocks.19–21 This is done by destructive and non-destructive methods; destructive methods directly measure biomass by harvesting the tree and measuring the actual mass of each of its components.22 They are very accurate but cutting down trees is both costly and time consuming.23 In contrast, indirect methods estimate biomass using biomass models and biomass expansion factors (BEFs) are inexpensive and time efficient. Moreover, research results in correlation to biomass and carbon stock in the country are scanty, little study and consistent monitoring systems has been implemented across intersite, intrasite and temporal variability of biomass estimation as compared to other tropical and subtropical countries.24–29
Gara-Mukitar forest is dry afromontane forest type found in Gemechis district and managed by Oromia Forest and Wildlife Enterprise which is advocating preservation and protection of the natural forest through participatory forest management approach. Accordingly, illegal cutting of trees in the natural forest is prohibited by the community bylaw developed by the enterprise and allowance of use for natural forest products is selectively applied based on age of tree and composition. The plantation forest is also developed and managed by enterprise. However, no studies have been conducted in investigating the carbon sequestration potential of the Gara-Muktar forest. Besides, there is scanty of information regarding appropriate forest management options to increase productivity and ensure sustainability of the forest. Thus, this study was hypothesized that there is special variation in carbon stock along the altitudinal gradients. Therefore, the study was aimed to evaluate carbon stock variations of Gara-Muktar forest along altitudinal gradients such as altitude to contribute and give some relevant information for local and regional administration, policy makers and other conservation organization.
Description of the study area
Geographical location: This study was conducted at Gara-Mukitar forest in Gemechis district of the West Hararghe zone of the Oromiya National Regional Stat, Eastern Ethiopia. Gemechis district is one of the 14 districts in West Hararghe zone which is located at 343km east of Addis Ababa and about 17km south of Chiro, capital town of the zone. It shares borders with Chiro district in the west and north, Oda Bultum district in the south and Mesala district in the east. The district covers an area of 77,785ha and 184,032 total population. Gara-Mukitar mountain forest is one of the remaining patches and disturbed dry afromontane forests in the region. This forest is located between the geographical coordinate 34°18'43"-43°04'33" E longitude and 10009'24"-30°18'43"N latitude with an altitude of 2200-3010 mabove sea level (Figure 1).
Climate
The district has a bimodal rainy season ranged from 1025 to 1200mm, with annual mean temperature ranging from 22.5-24.5°C. The main rain season is from June up to August and the short rain from February up to April. More than 75% of the total rain falls in June, July, August and September (locally known as kiremt season).30
Vegetation
The vegetation type of Gara-Mukitar mountain forest falls under dry afro-montane forest.31 The study area was dominated by Maesa lanceolata. Juniperus procera, Croton macrostachyus, Rhus glutinosa and Allophylus abyssinicus. There are also planted species such as:-Juniperus procera, Cupressus lusitanica, Hagenia abyssinicusand Croton macrostachyus.
Reconnaissance survey
A preliminary discussion forum was held with the higher officials of the Oromiya Wildlife and Forest Enterprise in Chiro, Western Hararghe Zone to aware them about the study and to collect secondary information about Gara-Mukitar forest. Subsequently, a reconnaissance survey was conducted through a field visit and physical observation across the Gara-Muktar forest patches and the surroundings. The study forest was classified into three forest strata, as pure natural forest, pure plantation and mixed natural plantation forest. Further, stratified based on species composition, diversity, structure, disturbances levels and accessibility. Then an inventory was done starting from top to bottom elevation gradients (altitude: Higher altitudinal gradient, 2950-2748m,Middle altitudinal gradient, 2748-2548m and Lower altitudinal gradient 2548-2348m).
Plot sampling
A systematic random sampling approach was implemented to conduct the inventory. A total of thirty six; 18 (20m x 20m) pure natural forest, 12 (20mx20m) natural mixed and 6 (20mx20m) pure plantation square sampled plots were marked out, based on the Neyman optimal allocation formula in the forest.22,32 The plots were laid out along 200m ground distance, starting from the highest ridges to the lowest ridges of the mountains’ using a measuring tape, GPS and compass. The boundaries of the main plots were pegged and marked, then altitude, slope, latitude and longitude data were recorded from the center of each main plot. A total of six transect lines were used along altitudinal gradient from top to bottom ridges of the mountain for data collection. The distance between two consecutive transect lines was 500m. Sampling and data collection were done in these measured plots.
Woody plant species sampling
Individual species were categorized into trees (≥5cm DBH), shrubs, saplings (height ≥1.3m) following the Lamprecht classification Lamprecht.33 Before conducting the actual measurements all bordering trees and shrubs were marked using red paints and numbered. Tree DBH (cm) was measured to the nearest two digits using a metallic caliper for smaller and medium sized trees, while for bigger trees measurement was made using diameter tape. Crown height, commercial height and total height (meter) were measured to the nearest two digits using Vertex III digital electronics tree height measurement instrument. In cases where trees branched at the breast height or below, diameter was measured separately for each branch. Likewise, the diameter at each stem was measured separately for trees with multiple stems connecting near to the ground. For irregularities and or buttresses on large trunks, measurement was taken at the nearest lower points. In similar way for each individual trees measurement were taken in the main plot. Height and diameter measurement were done using graduated wooden bars and metallic caliper, respectively.
Herb and grass (LHGs) carbon measurement
Destructive sampling method were used for litter, herbaceous and grass plants under 1mx1m, five subplots found in the main plot. Harvesting of litter, herbs and grasses were made. Fresh weights of all the collected subsamples were taken in the field. Then composited one hundred gram fresh herbaceous plant sub samples were taken into laboratory, oven dried at 70ºC for 24hours until constant weight. Organic carbon was estimated as 50% of the dry matter of herbaceous plants was considered as carbon. Similarly, the collected litter and twig samples were oven dried in the laboratory at 70ºC for 24hours and weighed, carbon was also taken as 50% of the dry matter of wood.
Soil sampling and analysis
Soil samples were collected within five 1m2 sub-plots in which LHGs samples were taken (Figure 2). A total of 360 samples (180mineral soil and 180cores) were collected for analyzing organic C%, and bulk density. Five hundred gram of composite soil sample was collected in each plot to determine organic carbon. All the collected soil samples were labeled and taken to the laboratory for analysis. Determination of percentage of carbon in soil was conducted in Haramaya University soil laboratory. Soil bulk density was calculated with a 5cm high cylinder that was introduced vertically in one sampling point for each depth interval. To determine SOC, moist soil samples was oven dried in the laboratory at 105°C for 12hours, re-weighted to determine moisture content and bulk density. Total organic carbon (%) was analyzed according to Walkley-Black's method following the procedure described in.34 Bulk density for each soil depth was determined as the ratio of mass of core sampled oven dry weight of dry soil to volume of 5cm diameter and 5cm height steel-cylinder following the procedure of Keeney and Nelson et al.35
Carbon stock estimation
Diameter and height measurements were converted into units of biomass using allometric equations.35 The overall carbon stocks of Gara-Mukitar dry Afromontane. forest were obtained by adding all the carbon stocks in different carbon pools together and extrapolating it into hectare bases and multiplying it with biomass expansion factor (BEF). Then it was converted into carbon using 50% of the dry biomass of an individual tree as carbon.7,28,,29,37,38
Above ground biomass
Above ground biomass was calculated using Ponce-Hernandez,39 (eq. 1) to estimate the AGB of the forest which relates DBH, tree height and wood specific density as dependent variables.
eq.1
Where AGBest, Above ground biomass (kg); D, DBH (cm); H, height (m); ρ, basic wood density (g cm-3)
Below ground biomass
Since direct measurement of BGB is expensive and time consuming task, it is derived from AGB (shoot root ratio). The BGB is 20% of AGB,8,39
eq.2
Where BGB, belowground biomass; AGB, aboveground biomass
Extrapolating carbon stocks from per plot basis into hectare basis requires the use of expansion factors. This standardization is required so that results can be easily interpreted and also compared to other studies. According to Pearson et al.40 the expansion factor is calculated as the area of a hectare in square meters divided by the area of the sample in square meter.
Litter, herb and grasses (LHGs) carbon
The harvested subsamples weighed and 100 sub samples were taken into laboratory, oven dried at 70ºC for 24 hours until constant dry weight. The litter biomass was calculated using the following formula Pearson et al.40:
eq.3
LB-Litter biomass (t C ha-1)
W, field-weight of wet field sample of litter sampled within an area of 1m2 (gm); W subsample (dry), weight of the oven, dry subsample taken to the laboratory (gm); W subsample (fresh), weight of the fresh sub-sample taken to the Laboratory (gm); A, Sampling area 1m by 1m.
Note: Carbon stock of the litter was taken as 50% of its dry biomass
Soil organic carbon estimation
The carbon stock density of soil organic carbon was calculated as Kidanemariam Kassahun et al.41 from the volume and bulk density of the soil.
eq.4
Where, V,Volume of the soil in the core sampler in cm3;h, the height of core sampler in (cm); r, the radius of core sampler in (cm). Moreover, the bulk density of a soil sample was calculated as follows:
eq.5
Where; BD, bulk density of the soil sample (gmcm3); Wav (dry), average air dry weight of soil sample (gm); V, volume of the soil sample in the core sampler (cm-3). Therefore, the carbon stock in soil was calculated as follows:
eq.6
Where; SOC, Soil Organic Carbon stock per unit area (t C ha-1); BD, soil bulk density (gmcm-3); d, the total depth at which the sample was taken (30cm) and%C-Carbon concentration (%).
Total carbon stockThe total carbon stocks (carbon density) were calculated by summing up all the carbon stocks of each carbon pools of the forest Pearson et al.40 The total carbon stock was then converted into tons of CO2 equivalent by multiplying it by 44/12, or 3.67 Pearson et al.40
Data of trees both aboveground and belowground, LHGs and soil carbon were processed using MS Excel spreadsheet and R version 3.4.2 for one-way analyses of variance (ANOVA) were used.
Floristic composition of Gara-Mukitar Forest
A total of 15 common families with 18 tree species were found in the study area and individual trees having DBH ≥5cm with total of 888 trees were recorded. Fabaceaewas the most diverse family, whileMaesa lanceolata. Juniperus procera and Rhus glutinosa were the most dominant species with their relative density of 20.65, 18.95 and 11.06% respectively (Table 1) (Figure 3). However, Vitexdoniana, Maytenus gracilipes and Ekebergiacapensiswere the least dominant ones with relative density of 0.11, 0.68 and 0.79% respectively. Maesa lanceolata, Croton macrostachyus and Juniperus procera were the most frequently occurred species while Scheffleraabssinica, Vernoniaamygdalina, Olea europaea, Cupressus lusitanica, Maytenus gracilipes and Ekebergiacapensiswerethe least occurred ones (Table 1) (Figure 3).

Figure 3 Tree density (upper left), relative frequency (lower) and relative density (upper right) of sampled tree species (%).
S.No |
Scientific name |
Local name |
Family name |
No. Sp. sampled |
No. of Plots. Spp. Occurred |
Density |
Frequency(F) |
||
(Stem /ha) |
R.D (%) |
Occurred in plot (%) |
R.F (%) |
||||||
1 |
Allophylus abyssinicus |
Ribiqa |
Santalaceae |
59 |
20 |
40.97 |
6.66 |
55.56 |
9.48 |
2 |
Bersama abyssincus |
Lolchisa |
Sapindaceae |
25 |
9 |
17.36 |
2.82 |
25.00 |
4.27 |
3 |
Croton macrostachyus Del. |
Bakanisa |
Eurphorbiaceae |
62 |
27 |
43.06 |
7.00 |
75.00 |
12.80 |
4 |
Cupressus lusitanica Mill |
Gattira -faranjii |
Cuppressaceae |
14 |
3 |
9.72 |
1.58 |
8.33 |
1.42 |
5 |
Dichrostachys cinerea |
Katame(Hatt) |
Fabaceae |
59 |
12 |
40.97 |
6.66 |
33.33 |
5.69 |
6 |
Dombeyatorrida P. Bamps |
Danissa |
Sterculiaceae |
37 |
13 |
25.69 |
4.18 |
36.11 |
6.16 |
7 |
Dovyalisabyssinica |
Koshim |
Flacourtiaceae |
31 |
10 |
21.53 |
3.50 |
27.78 |
4.74 |
8 |
EkebergiacapensisSparrm |
Sombo |
Meliaceae |
7 |
3 |
4.86 |
0.79 |
8.33 |
1.42 |
9 |
Erythrina abyssinica.htm |
Welenso |
Fabaceae |
34 |
10 |
23.61 |
3.84 |
27.78 |
4.74 |
10 |
Hagenia abyssinica (Bruce J.F Gmel.) |
Hexo |
Rosaceae |
42 |
7 |
29.17 |
4.74 |
19.44 |
3.32 |
11 |
Juniperus procera Hochst.ExA.Engl. |
Gaanttirahabiyya |
Cuppressaceae |
168 |
25 |
116.67 |
19.0 |
69.44 |
11.85 |
12 |
Maesa lanceolata.html |
Abiye |
Myrsinaceae |
183 |
29 |
127.08 |
20.7 |
80.56 |
13.74 |
13 |
Maytenus gracilipes Exell |
Kombolcha |
Celastraceae |
6 |
3 |
4.17 |
0.68 |
8.33 |
1.42 |
14 |
Olean europaea |
Ejersa |
Oleaceae |
11 |
4 |
7.64 |
1.24 |
11.11 |
1.90 |
15 |
Podocarpus falcatus (Thmb.) R.B. Ex. Mirb |
Birbirsa |
Podocarpaceae |
36 |
15 |
25.00 |
4.06 |
41.67 |
7.11 |
16 |
Rhus glutinosa |
Waka |
Fabaceae |
98 |
16 |
68.06 |
11.1 |
44.44 |
7.58 |
17 |
Vernonia amygdalina Del |
Ebicha |
Asteraceae |
13 |
4 |
9.03 |
1.47 |
11.11 |
1.90 |
18 |
Vitex doniana |
Juwaelo |
Lamiaceae |
1 |
1 |
0.69 |
0.11 |
2.78 |
0.47 |
Table 1 The names of tree species with their density and frequency in the study area (one way anova)
There was lower floristic composition and diversity in Gara-Muktar forest, this might be due to the agro-climatic locations of study site and a biotic factors. This result was in line with the findings of Patrick et al.7 and Kidanemariam Kassahun et al.41 The increasing and decreasing frequency and numbers of an individual species responds to a changing environment encountering one another at spatial scale. According to Zomer et al.42 forests are declining at alarming rate resulted in temperature change, land instability, soil and biodiversity and local people’s dependency for livelihood.
Vegetation structure of GaraMuktar forest was dominated by Maesa lanceolata, Juniperus procera and Rhus glutinosa trees. The vegetation structure of our study area showed inverse J-shaped distribution, which indicates greater potential of regeneration capacity of young (low DBH) trees, growing much faster having higher capacity in accumulating large carbon. The result was in line with the study conducted by several authors in the country. The number of species found in Gara-Mukitar forest was 18 which is lower than species reported in Munessa-Shashemene State Forest i.e. 36 by Delnatte et al.43 and Ades forest, Western Hararghe i.e. 44 by Zome ret al.42 This could be due to the variation of agro-ecological zone and human disturbances among those study sites of forests. The density of trees ha-1 (Table 1), in present study was almost in agreement with Guangua Ellala Forest.44
Trees species biomass carbon stock contribution of study area
The biomass carbon stock of tree was varied from one tree species to the other. Croton macrostachyus (25.83%) and Juniperus procera (21.23%) sequestered the largest portion of the forest carbon whereas Vernoniaamygdalina(0.15%) and Olea europaea(0.23%) sequestered the least biomass carbon stock (Figure 4).
Carbon Stocks along altitudinal gradient
The total aboveground biomass carbon stock density varied from 102.13±31.16 to 214.73±54.73t C ha-1 in higher and lower altitudinal gradient respectively (Table 2) (Figure 5). In addition, the mean total belowground biomass carbon stock density ranged from 42.94±10.94 to 20.42±6.23t C ha-1 in higher and lower altitudinal gradient, respectively. The mean total litter carbon density ranged from 1.06±0.46 to 3.64±1.41t C ha-1 in lower and higher altitudinal gradients (Table 2) (Figure 5). The mean total Litter carbon stock density ranged from 1.03±0.46 and to 3.64±1.41t C ha-1 in the lower and higher altitudinal classes respectively (Figure 5d). Similarly, the mean total soil carbon stock density ranged from 58.03±7.56 and to 156.13±45.64t C ha-1 in the lower and higher altitudinal classes respectively. There was weak negative correlation (R²=-0.4575) (Figure 6) between aboveground biomass carbon stock and altitude. Total belowground biomass carbon stock has also showed similar trends (R²=-0.4575), (Figure 6). The relationship between litter biomass carbon stock and altitude has also showed weak positive correlation (R²=0.3646), (Figure 6). Moreover, the soil organic carbons with corresponding altitude were regressed linearly.
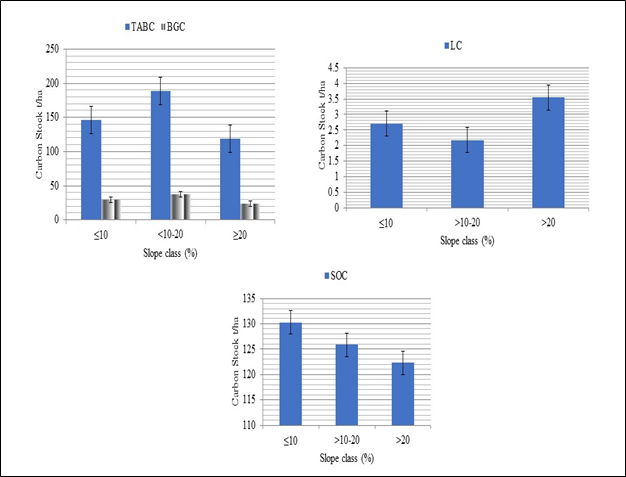
Figure 5 Mean Carbon stocks (t C ha-1) in different carbon pools Trees-TAGC and TBGC, (LHGs) and SOC with altitudinal classes.
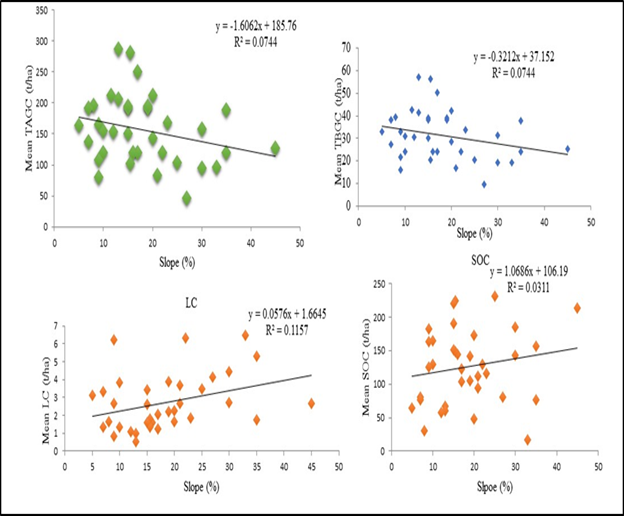
Figure 6 The correlation of carbon stock of different carbon pools TAGC, TBGC of trees, C (LHGs) and SOC with altitudinal gradient.
Gradient |
Carbon Stock |
|||
Altitude |
AGC |
BGC |
C(LHGs) |
SOC |
F |
19.400 |
19.4 |
6.18 |
6.415 |
P |
.0001 |
.0001 |
.005 |
.004 |
Table 2 Summarized results of one way ANOVA of correlation between different carbon stocks with altitude
Where AGC, above ground carbon; BGC, below grown carbon; LHGS, Litter carbon; SOC, soil organic carbon
With increasing altitude, the mean above and below ground biomass carbon showed a decreasing trend while litter carbon and soil organic carbon showed relatively an increasing trend. This is might be due to the absence of tallest trees with maximum DBH in higher elevation gradient. In high altitude areas the species richness of plants and their diversity of small size (low DBH) Fabaceae family dominant with altitude while, large size DBH woody plants decrease with altitude. This is might be due to multitude factors which vary with the altitude including the geomorphologic factors, soil, humidity, cloudiness, temperature Mohammed Gedefaw et al.45 and dissimilarity with the study site.46–48 In addition, due to the presence of greater numbers of farming communities living around the forest adjacent whose livelihoods depend on the forest product. This implies continuous removal of fallen litter, dead wood and twigs combined with illegal cutting for charcoal making, construction purpose, agriculture and livestock grazing could affect the balance of forest carbon stock.45 Another possible reason was also affirmed by Mohammed Gedefaw et al.46 as climatic factors can affect forest carbon stock with elevation gradient. Maximum LHGs carbon was stored in the upper followed by middle and lower altitudinal class. This might be due to the upper forest composition is natural forest while the middle is mixed and lower is plantation at adjacent land which was disturbed by human interaction. Similar result was reported by Belay Melese et al.26 The mean carbon density in soil organic carbon pool of the present study showed an increasing trend with the altitude (Figure 5). This is might be due to decreasing temperature and increasing precipitation. In addition, human pressure might be a confounding factor when analyzing the effect of elevation on SOC in forests as similar results were also reported by Belay Melese et al.26 and Twongyirwe et al.49 The carbon pools in the forest revealed significantly difference between with altitude, similar to this study; the results of studies conducted by different authors,45,44,48 also indicated significant differences between the carbon pools at elevation gradient (Table 2).
Analysis of variation of carbon stock in different carbon pools of Gara-Mukitar forest responded different carbon storage capacity along altitudinal gradients. Maesa lanceolata, Juniperus procera and Rhusglutinosa are the dominant species in the area. The study forest was mostly dominated by small sized tree species while tree species having lower range of diameter possess more density than higher diameter class. The amount of carbon stock per species was varied where the highest carbon stock was recorded for Scheffleraabssinica, followed by Podocarpusfalcatus. The total carbon stock of the study forest was 316.6t C ha-1 of carbon and 1161.59t C ha-1 of CO2 eq. The aboveground and belowground carbon density, litter and soil carbon density showed distinct patterns along altitudinal gradient and thus the forest has significant role in carbon sequestration. The present study was limited to carbon stock estimation thus, further studies on composition, diversity, structure of woody plants and land-use management system in the study area are recommended. Data from this research can be used as a benchmark for other scholars to conduct further study. Therefore, the result of the current finding together with other results on forest carbon stock would also serve as a potential entry point for the engagement of the forest in REDD+project.
None.
The authors' thanks West Harargea, Oromiya Forest and Wild Life Enterprize, for assisting us in field data collection and preparation of plant and soil samples. The School of Natural Resource Management and Environmental Science,Haromaya University, for hosting AsaminewWodajo and the Ethiopian Environment and Forest Research Institute (EEFRI), headquarter, for covering the cost of fieldwork and laboratory analysis. More over all the authors declared that this manuscript is our original work and no competing claims among the authors.
Author declares that there is no conflicts of interest.

©2020 Wodajo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.