eISSN: 2577-8307


Research Article Volume 2 Issue 2
1Department of Zoology, Feroze Gandhi P.G. Degree College, India
2School of Environmental Sciences, Jawaharlal Nehru University, India
Correspondence: Tuneera Bhadauria, Department of Zoology, Feroze Gandhi College, Raebareli, Uttar Pradesh, India, Tel 91 9453022105
Received: November 22, 2017 | Published: March 7, 2018
Citation: Bhadauria T, Saxena KG. Community structure and recolonization by earthworms in rehabilitated ecosystems in garhwal himalayas, India. Forest Res Eng Int J. 2018;2(2):35-47. DOI: 10.15406/freij.2018.02.00024
Consequences of deforestation arise from site degradation leading to modification of soil properties this significantly affect both incidence and abundance of soil macro fauna. Earthworm communities are more directly altered by these changes. Endemic and exotic species co-existed in the study area following deforestation and intensive cultivation. That native species were dominant in the undisturbed sites and disturbance and degradations leads to invasion by the exotic species holds true in our study. The sites under study represented the degraded areas as none of the species reported from the present experimental plots were endemic to the region, all the species are either peregrine exotic or peregrine endemic to the area as many of the endemic species of this region probably exterminated during the last Quaternary Glaciation. The numbers of species present at our sites ranged between 2 to 5. The presence of litter layer and lower perturbation pressure resulted in the numerical dominance of Lennogaster pussilus in the Oak forest (OF) and the higher biomass of A. alexandri may be because of the larger size of the earthworm. B parvus, L. pussilus and P. excavatus are litter-associated taxa which were more directly affected by OF clearance and the resulting decrease in available litter, thus explaining their disappearance in the changed ecosystems, however improved soil moisture and temperature as well as input of organic matter in rehabilitated agricultural land (RAL) could probably be favourable factor for decolonization anddominance of L. pussilus. A. alexandri had wider ecological amplitudeoccurring under all land use types as reported in our studies, conversion of two other land use types resulted in lower species diversity. Our results show that the abandoned agriculture land (AAL) remains closer to the AL(T) than to the forest because in these land use types the overall vegetation diversity remains low corresponding to a low diversification of the organic resources thus explaining the similarity between the earthworm communities in land use types. Seasonal rhythmic pattern was exhibited by all the species identified.
Keywords: deforestation, rehabilitation, earthworm community, functional guild, ecological categories, diversity index
Himalaya is a vast and diverse mountain system. Agro forestry land use covering (20%) of geographical area of Indian Himalayas is distributed as patches in the matrix of forest covering (52%) area. Forest based tree- crop livestock integrated farming is the predominant traditional land use in central Himalayas.1 Hill agriculture appears to be key threat to soil biodiversity and ecosystem service due to huge amount of biomass extraction to sustain live stock and produce manure for managing soil fertility and also in terms of direct loss of forest cover due to agriculture land use.2 This problem has been further accentuated by extensive deforestation and unsustainable land use causing extensive degradation of the mountains. A pilot project was carried out to restore and rehabilitate the abandoned agriculture land site and degraded forest land site through agro forestry and forestry plantations.3 Land degradation implies a decline in the useable natural resource base and therefore loss of biodiversity and ecosystem functions. The interacting functions of soil organisms and the effects of human activities in managing land for agriculture and forestry affect soil health and quality.4 Soil faunal biodiversity is an important resource for environmental monitoring and natural resource management, changes in the variety and abundance of organisms in response to ecosystem disturbance, degradation and rehabilitation provide important management information.5 Soil management options can have dramatic effects upon soil invertebrate communities.6,7 Earthworm communities are the result of both interactions between species8 and sensitivity to ecological factors9 presence or absence of ground vegetation and changes in its composition are known to affect the composition of earthworm communities.10 This is the first systematic study where work is focused primarily on the ecological impact, the extent, causes and consequences of varying land degradation and subsequent rehabilitation strategies on earthworm fauna in Central Himalayas. Micro scale variability in earthworm community in traditional agro ecosystem will also be focused by comparing two types of micro sites viz below tree canopy from (1-3m) the bole and outside tree canopy (1-3m) from canopy margin. Changes in the vegetation cover (forest clearance, tree plantation) change the soil microclimate condition therefore present study also aims to analyze how these changes affected the functional guild of the earthworms.
Soil fauna vary through time as they have seasonal rhythms mainly regulated by temperature and moisture and thus constitute one of the important factors of changes in the species assemblage structure.11,12 Consequently, the way seasonal variations of earthworm communities impacted by land-use changes occurs as well as its impact on biomass and density of earthworms have been examined across the sampling sites.
The study was carried out at Banswara village located at 1200m above mean sea level in Chamoli district, Garhwal (latitude 30°27’N and 79°5’E). The climate is typical monsoon, monthly minimum and maximum temperature varying in the range of 6-21°C and 18-35 °C, respectively, with an average annual rainfall of 1700mm. 80% of total rain fall is received during the monsoon period of July to September (Figure 1A) (Figure 1B). Soil moisture and temperature are as presented in Figure 2(A) & Figure 2(B). The soil is sandy loam to loamy sand in texture (Table 1) derived from felspathic quartz schists ,quartz muscovite schists and quartz chlorite schist.13 To study the impact of changed land use practices on earthworms following land uses were identified in the village Banswara.
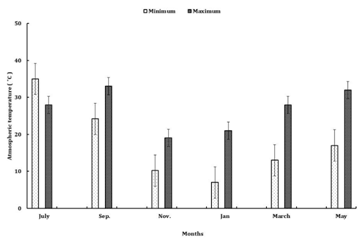
Figure 1(A) Monthly maximum and minimum atmospheric temperature for study area in Garhwal Himalayas during the study period from July 2008-2009.
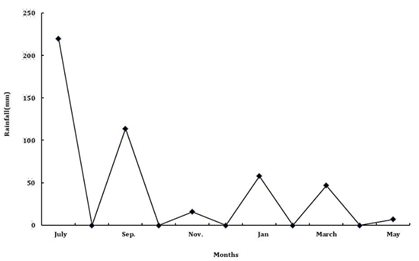
Figure 1(B) Monthly Rainfall for study area in Garhwal Himalayas during the study period from July 2008-May 2009.
The reserved forests are unmanaged old-growth sub tropical warm temperate forest dominated by Quercus samicarpifolia, Q.floribunda Cedrus .
The farming system is characterized by settled rainfed organic agriculture on terraced slope with scattered multipurpose trees
The sampling of soil fauna was done below was done below the tree canopy (1-3mdiameter from the bole).
The sampling of soil fauna was done below was done below the tree canopy (1-3m from canopy margin).
Degraded abandoned agriculture land represented the agriculture terraces which had been abandoned due to poor soil fertility status, they were degraded and exposed to uncontrolled grazing.
The rehabilitated land site was the abandoned agriculture land site where the terraces were repaired and agricultural crops were grown providing supplemental irrigation and organic manure along with the planted trees following traditional farming practices. This site was protected from grazing and other human disturbances.
Degraded Forest Land represented degraded community forest land which had negligible tree cover and was exposed to uncontrolled grazing. Soils on the degraded land were considerably eroded with respect to abandoned agriculture land.
The Rehabilitated forest site represented the plantation of trees on degraded community forest land which had more stressful environment as compared to the RAL. This site was protected from grazing and other human disturbances.
Where = number of individuals of species i in the sample. N=total number of individuals in the sample.
S= Number of species in the sample. 1-D^ = Simpsons Index of Diversity
Where
Xij is the number of individuals of species i in group j
Xik is the number of individuals of species i in group k
N is the total number of individuals in the sample j and k
Soil characteristics varied under different land use types, except for RFL soil pH was mildly acidic in all other sites, soil bulk density was higher in AAL and DFL when compared to other land use types under study. Soil organic C (F0.05,6,14= 32.9) and Nitrogen (F0.05,6,14= 151) varied significantly between different sites, organic C was significantly higher (F0.05,6,14= 151) in AL (T) as compared to all other sites but did not vary significantly between RFL and OF, between AL (WT) and OF. Total nitrogen was significantly higher (F0.05,6,14= 151) in AL (WT) as compared to all other sites, but it did not vary significantly between AL (WT) and RFL and between AL (T) and OF. Soil organic carbon (q0.05,21,3 = 3.41) and nitrogen (q0.05, 21,3= 4.76) was significantly higher in AAL as compared to DFL (Table1).
A total of eight species belonging to four families were recorded from land use types under study and the selected attributes of sampled earthworm species (Table 2). All the earthworm species recorded across different land use types were both peregrine endemic or peregrine exotic to the region, none of these were endemic to Garhwal Himalayas (Table 2).
Earthworm species richness did not vary significantly between the OF, AL (T) and AL (WT), but in AAL and DFL number of species was lower; however rehabilitation of these ecosystems led to increase in species number in RAL and RFL (Table 3).
Three peregrine exotics Amynthas alexandri, Bimastos parvus, Metaphire anamola and two peregrine native species Lennogaster pussilus, Perionyx excavatus were present in the OF .Conversion of the forest to agro ecosystems led to the change in the community structure with the loss of exotics Bimastos parvus and natives Lennogaster pussilus, Perionyx excavatus in AL (T) and AL (WT), but recolonisation by exotic Metaphire birmanica, Octochaetona beatrix and natives Drawida nepalensis occurred here. M. anamola, M. birmanica and D nepalensis were present in AAL, however rehabilitation of degraded ecosystems RAL resulted in loss of M. birmanica and recolonisation by L. pussilus and A. alexandri. Only exotic A. alexandri and M. birmanica were present in DFL, but A. alexandri, M. anamola and M. birmanica .were present in RFL (Figure 3). B. parvus and P. excavatus did not recolonize any rehabilitated ecosystems whereas A. alexandri and M. anamola were absent in RAL and DFL though they were present on all the other sites under study (Table 4).

Figure 3 Changes in earthworm species composition across different land use types in Garhwal Himalayas.
Total density (F0.05,6,14= 228.24) as well as biomass (F0.05,6,14= 403.78) of earthworm’s varied significantly between different land use types due to changes in land use practices. Conversion of forest to agro ecosystem resulted in significantly higher density (q,0.05,,14,7= 122.73) and biomass (q,0.05,14,7= 109 values ) in AL(T). Density (q,0.05,14,4= 114) and biomass of earthworms (q,0.05,14,4= 112) also increased significantly in rehabilitated ecosystems RAL and RFL when compared to AAL and DFL (Table 5).
The density and biomass of exotic peregrine earthworm species varied significantly between different sites (Figure 4A) (Figure 4B). These values were significantly higher in AL (T) followed by AL (WT) (q0.05,12,6= 102) and did not vary significantly between RAL, DFL and AAL. The biomass values of exotic species also did not vary significantly between OF and RFL. The density (F0.05,6,14= 211) and biomass (F0.05,6,14= 248) of endemic peregrine species also varied significantly between different sites and were significantly higher in AL(T) as compared to other sites. The biomass values of endemic species did not vary significantly between OF and AAL.
Within the same sites the density of exotic and endemic peregrine earthworm species did not vary significantly in OF, but exotic species had higher biomass (t(2)0.05,4)=7.5 as compared to endemics species. Exotic earthworm species were significantly more abundant (t(2)0.05,4= 57.32) with higher biomass (t(2)0.05,4= 25.5) values in AL(T) than endemic species. In AL (WT) and in AAL endemic species were more abundant numerically and had significantly higher biomass value than exotics species. Endemic species had significantly higher abundance in RAL (t(2)0.05,4= 5.75) as compared to exotic species but biomass values of exotic and endemic earthworm species did not vary significantly in here. Endemic species were absent in DFL and RFL.
Functional guild diversity varied under different land use types (Figure 5A) (Figure 5B), all the three functional categories were present in RAL. Anecics were absent in OF and AAL, in AL (T), AL (WT), DFL and RFL epigeic species were absent, where as in AAL only endogeics species were present. Epigeic were significantly more abundant (F0.05,2,6= 12.23 )with higher biomass values (F0.05,2,6= 7.54 ) in OF. Both endogeics and anecics earthworm species were significantly more abundant (F0.05,2,6= 58.54) and had higher biomass values (F0.05,2,6= 42.63 ) at AL(T) compared to all other sites.
Within the same site, numerically epigeics formed the dominant (F0.05,2,6= 37.19) functional group under OF and in RAL (F0.05,2,6= 18.54), whereas endogeic were dominant under AL(T) (F0.05,2,6= 11.7), AL(WT) (F0.05,2,6= 28) and RFL(F0.05,2,6= 7.54), in AAL they were the only functional group present. Endogeics had significantly higher (F0.05,2,6= 19.4) biomass in OF and RAL when compared to epigeic and anecics. Anecics had lower abundance and biomass compared to other functional groups within the same sites.
Seasonal variation for different earthworm species abundance and biomass (Figure 6 (A-H)).
Alexandri All the species showed significant seasonal variation. The density and biomass varied significantly seasonally in OF (F0.05,5,12= 124.6), AL(T) (F0.05,5,12= 133.8), AL(WT) (F0.05,5,12= 23.04) and RFL (F0.05,5,12= 8). It did not vary significantly in RAL and DFL. The species had significantly higher population density (q0.05,12,6= 7.54) and biomass (q0.05,12,6= 8) during Sep at all sites except in AL(WT)and where it had higher biomass (q0.05,12,6= 7.34) during July and in RFL where species showed a minor increase in population density during January. B. parvus was present only in the oak forest and did not show any significant variation seasonally in density as well as biomass values.
The Simpsons Diversity Index within the earthworm communities under different land use types was lowest in AAL and maximum in OF. The diversity index was similar between OF and RAL. Within the agro ecosystem the diversity Index was lower in AL (WT) compared to AL (T), but rehabilitation of degraded ecosystem led to improved diversity Index in RAL and RFL (Figure 7).
L. pussilus was dominant numerically in OF but A. alexandri had higher biomass values as compared to other species. At AL(T), AL(WT) and AAL D. nepalensis was dominant numerically as well having a higher biomass percentage, L. pussilus was dominant numerically at RAL but D. nepalensis had higher biomass here. A. alexandri was dominant numerically as well as higher biomass values in DFL and RFL but M. anamola had higher biomass percentage at the RFL site (Table 6).
Morrisitas index of similarity indicated that the earthworm community in AL (T) was more similar to AAL than AL (WT). Rehabilitation of degraded agro ecosystem showed that the community structure of RAL was more similar to the OF where as community structure between RFL and DFL were closer to one another (Table 7).
A significant correlation coefficient was observed between earthworm population and soil moisture and temperature. A. alexandri was positively correlated to soil moisture ( ) and temperature ( ). In AL(WT), RAL, DFL. B. parvus, and L. pussilus showed positive correlation ( ) to soil moisture and temperature, P. excavatus was positively correlated to moisture ( ) but did not show any co relation to soil temperature. D. nepalensis was positively related ( ) to soil moisture in AL (T), AL (WT) and RAL but it did not show any correlation with soil moisture in AAL. M anamola was positively related to soil moisture ( ) and temperature ( ) in AL (WT) AAL and RAL but in AAL it did not show any correlation to soil moisture. In AL (WT) and AAL M. birmanica exibited similar trend as M. anamola however in DFL ( ) and RFL ( ) it showed positive correlation to soil moisture only. O. beatrix showed positive correlation to soil temperature ( ) in AL (T), AL (WT), but it did not show any relation to soil moisture (Table 8).
Land use/cover |
Soil property |
|||
|---|---|---|---|---|
pH |
Bulk density |
Organic carbon (g kg-1) |
Total nitrogen (g kg-1) |
|
(g cm-3) |
||||
Oak forest (OF) |
6.1a |
1.11a |
12.6a |
1.2a |
Microhabitat below agro forestry tree patches AL(T) |
6.4a |
1.04a |
18.1b |
0.9a |
Microhabitat without agro forestry tree patches (ALWT) |
6.2a |
1.10a |
13.2a |
1.9b |
Abandoned agricultural land(AAL) |
6.4a |
1.4b |
8.6c |
0.8c |
Rehabilitated agricultural land (RAL) |
6.3a |
1.12a |
15.0d |
1.4d |
Degraded forest land(DFL) |
6.2a |
1.42b |
8.3c |
0.7c |
Rehabilitated forest land (RFL) |
5.8b |
1.14a |
12.4a |
1.7b |
Table 1 Soil characteristics of different land use/cover types in central Himalayan ( ), India (Average mean values for one year. Values followed by different superscript letters are significantly ( ) different.
Species |
Color |
Length*(mm) |
Diameter(mm)* |
Family |
Peregrine Species |
Native region |
Ecological Catagories |
Amynthas alexandri |
Dark Brown |
180 |
8 |
Megascolecidae |
Exotic |
S.E.Asia |
Endogeic |
Bimastos parvus |
Dark brown |
70 |
2 |
Lumbricidae |
Exotic |
N.America |
Epigeic |
Drawida nepalensis |
Light whitish |
140 |
7 |
Moniligastridae |
Native peregrine |
Eastern Himalayas |
Endogeic/Anecic |
Lennogaster pussilus |
Light brown |
50 |
2 |
Octochaetidae |
Native peregrine |
Middle and Upper Gangetic plains |
Epigeic |
Metaphire anamola |
Dark brown |
140 |
4 |
Megascolecidae |
Exotic |
S.E.Asia |
Endogeic |
Metaphire birmanica |
Blackish brown |
160 |
4 |
Megascolecidae |
Exotic |
Burma |
Endogeic |
Octochaetona Beatrix |
Light Pink |
120 |
4 |
Octochaetidae |
Native peregrine |
Eastern and Western Plateau region |
Endogeic |
Perionyx excavatus |
dark violet /Purple |
76 |
3 |
Megascolecidae |
Native peregrine |
Eastern Himalayas |
Epigeic |
Table 2 Selected attributes of sampled earthworm species across different land use types in Garhwal Himalayas
Measurements of largest individuals indicating the maximum achievable size
Land Use Pattern |
Species Number |
Old secondary Forest OF |
5 |
Microhabitat below agro forestry tree patches AL(T) |
5 |
Microhabitat without agro forestry tree patches AL(WT) |
5 |
Abandoned Agriculture LandAAL |
3 |
Rehabilitated Agriculture LandRAL |
4 |
Degraded Forest Land DFL |
2 |
Rehabilitated Forest Land RFL |
3 |
Table 3 Effect of land use patterns on earthworm species richness across different land use types in Garhwal Himalayas
Species |
OF |
ALT |
ALWT |
AAL |
DFL |
RAL |
RFL |
Amynthas alexandri |
Y |
Y |
Y |
N |
Y |
Y |
Y |
Bimastos parvus |
Y |
N |
N |
N |
N |
N |
N |
Drawida nepalensis |
N |
Y |
Y |
Y |
N |
Y |
N |
Lennogaster pussilus |
Y |
N |
N |
N |
N |
Y |
N |
Metaphire anamola |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Metaphire birmanica |
N |
Y |
Y |
Y |
Y |
N |
Y |
Octochaetona Beatrix |
N |
Y |
Y |
N |
N |
N |
N |
Perionyx excavatus |
Y |
N |
N |
N |
N |
N |
N |
Table 4 Occurrence of earthworm species across different land use types in Garhwal Himalayas
Density(individuals/m2/year) |
Biomass(g/m2/year) |
|
OF |
a97±18 |
a 79±2.7 |
AL(T) |
b358±25 |
b 663±35.74 |
AL(WT) |
c 263±21 |
c 403±16±37 |
AAL |
d 19±1.4 |
d 27±2.4 |
RAL |
e 59±1.8 |
e 50±3.2 |
DFL |
f 7±3.4 7 |
f 15.4±1.2 |
RFL |
d 28±1.1 |
g 62.5±4.1 |
Table 5 Density (Individuals/m2/year) and biomass (g/m2/year) of earthworm species across different land use types in Garhwal Himalayas. Numbers followed by the same letter are not significantly different ( ).
OF |
AL(T) |
AL(WT) |
AAL |
RAL |
DFL |
RFL |
|
A. alexandri |
16.5 |
11.84 |
4.57 |
0 |
12 |
*57.15 |
*36 |
(*39) |
(12.7) |
(6) |
(19) |
(*57) |
(29) |
||
B.parvus |
7.22 |
0 |
0 |
0 |
0 |
0 |
0 |
(3.1) |
(0) |
(0) |
(0) |
(0) |
(0) |
(0) |
|
D. nepalensis |
0 |
*40 |
*44.87 |
*61 |
21 |
0 |
0 |
0 |
(*38) |
(*53) |
(*56) |
(*41) |
0 |
0 |
|
M.anamola |
26.8 |
16.9 |
5.71 |
12 |
8 |
0 |
32.14 |
(32) |
(16) |
(6) |
(24) |
(16) |
(0) |
(*39) |
|
M.birmanica |
0 |
30.71 |
8.37 |
27 |
0 |
42.86 |
32.14 |
(0) |
(33) |
(11) |
(20) |
(31) |
(43) |
(32) |
|
L.pussilus |
*32.99 |
0 |
0 |
0 |
*59 |
0 |
0 |
(31) |
(0) |
(0) |
(12) |
(0) |
(0) |
||
O.beatrix |
0 |
0.56 |
36.5 |
0 |
0 |
0 |
0 |
(0) |
(0.3) |
(24) |
(0) |
(0) |
(0) |
(0) |
|
P.excavatus |
16.5 |
0 |
0 |
0 |
0 |
0 |
0 |
(10.1) |
(0) |
(0) |
(0) |
(0) |
(0) |
(0) |
Table 6 Earthworm species dominance (%) Abundance & Biomass (Values in parenthesis) within the same land use types in Garhwal Himalayas
OF |
AL(T) |
AL(WT) |
AAL |
RAL |
DFL |
RFL |
|
OF |
1 |
0.248 |
0.08 |
0 .088 |
0.72 |
0.252 |
0.51 |
AL(T) |
1 |
0.688 |
0.91 |
0.32 |
0.491 |
0.625 |
|
AL(WT) |
1 |
0.75 |
0.273 |
0.181 |
0.15 |
||
AAL |
1 |
0.315 |
0.24 |
0.311 |
|||
RAL |
1 |
0.142 |
0.175 |
||||
DFL |
1 |
0.81 |
|||||
RFL |
1 |
Table 7 Morisita’s index of similarity for earthworm communities between different land use types
|
A. alexandri |
B.parvus |
D. nepalensis |
M.anamola |
M.birmanica |
L.pussilus |
O.beatrix |
P.excavatus |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
M |
T |
M |
T |
M |
T |
M |
T |
M |
T |
M |
T |
M |
T |
M |
T |
OF |
-0.03 |
0.30 |
**0.96 |
**0.95 |
A |
A |
0.34 |
0.41 |
A |
A |
*0.89 |
*0.87 |
A |
A |
**0.92 |
*0.9 |
AL(T) |
-0.03 |
0.40 |
A |
A |
*0.88 |
*0.84 |
**0.97 |
0.55 |
**0.98 |
*0.96 |
A |
A |
0.62 |
**0.92 |
A |
A |
AL(WT) |
*0.88 |
*0.87 |
A |
A |
*0.85 |
*0.87 |
**0.99 |
*0.87 |
0.56 |
0.18 |
A |
A |
0.58 |
**0.95 |
A |
A |
AAL |
A |
A |
A |
A |
0.19 |
*0.81 |
**0.96 |
*0.97 |
**0.97 |
*0.95 |
A |
A |
A |
A |
A |
A |
RAL |
**0.98 |
*0.95 |
A |
A |
*0.88 |
*0.83 |
**0.98 |
*0.96 |
A |
A |
*0.85 |
*0.89 |
A |
A |
A |
A |
DFL |
**0.97 |
*0.96 |
A |
A |
A |
A |
A |
A |
**0.97 |
A |
A |
A |
A |
A |
A |
A |
RFL |
0.31 |
0.27 |
A |
A |
A |
A |
-0.28 |
-0.35 |
*.85 |
-0.38 |
A |
A |
A |
A |
A |
A |
Table 8 Correlation coefficient(r) for soil moisture (M%); temperature (T (°C) and earthworm species abundance in Garhwal Himalaya
*indicates
, **indicates
Consequences of deforestation arise from site degradation leading to strong modification of soil properties this in turn can significantly affect both incidence and abundance of soil macro fauna. Earthworm communities are more directly altered by this changes.19 Endemic and exotic species co existed in the study area following deforestation and intensive cultivation. That native species dominate the undisturbed sites and disturbance and degradations leads to invasion by the exotic species20 holds true in our study. The sites under study represented the degraded areas as none of the species reported from the present experimental plots were endemic to the region, all the species are either peregrine exotic or peregrine endemic to the area as many of the endemic species of this region probably exterminated during the last Quaternary Glaciation.21 The elimination of old secondary forest and its replacement with agroecosystem also led to changed species composition due to altered habitat with peregrine exotics M. birmanica, O. beatrix and peregrine natives D nepalensis replacing peregrine exotics B. parvus, and natives L. pussilus and P. excavates in AL (T) and in AL (WT), similar results have also been shown through the studies.22 Extreme degradation of agriculture ecosystems due to faulty land management practices probably led to the loss of peregrine exotics A. alaxandri, M. anamola and O. beatrix in AAL as has also been shown through the studies19 in land use types in Northern Kwazulu Natal South Africa. The disappearance of peregrine exotics A. alaxandri and peregrine natives L. pussilus in RAL could probably be related to changed vegetation and edaphic conditions in RAL as has also been reported by Fragoso C et al.23 for earthworm communities in disturbed natural systems of tropical east Mexico and rehabilitation of RAL through various trees and crops probably led to invasion of peregrine exotic M. birmanica and M. anamola in these area. Higher level of degradation leading to replacement of OF to degraded forest land led to loss of exotic B. parvus, M. anamola and natives L. pussilus, P. excavatus. Resistance to invasion by endemic species in DFL and RFL could be a function of physical and chemical characteristics of the site24 and the unsuitability of the habitat probably impeded the invasion by the endemic species in the ecosystems under study.25 The absence of original species composition in RAL and RFL as compared to OF even after a period of 15 to 20 years suggest that this is probably still the secondary successional stage over a time scale of 15 years.26
Land-use alteration generally results in changes of vegetation and these changes have a significant effect on soil macrofauna.4 Study done by19 in South Africa showed most land uses supported between five and seven species. but the number of species present at our sites were lower ranging between 2 to 5. The statement that earthworm populations in cultivated lands are generally lower than those found in undisturbed habitats27 does not hold true in our studies, the traditional agriculture probably have a positive effect on earthworms through improved food supply as a result of recycling of crop residues, and organic manure added to the soil resulting in loosening of soil to an extent that facilitates burrowing by earthworms.28 This also explains the increased abundance and biomass of earthworm in AL(WT) as compared to OF. With lower bulk density and higher carbon percentage, coupled with reduced disturbance this effect was more prominent in AL(T). In RAL the supplemental irrigation and organic manure along with the planted trees following traditional farming practices favored the increase in total earthworm density as well as biomass. The smaller community under AAL and DFL than OF is attributable to loss of species when the land was cleared and cultivated. More stress full environment in DFL as compared to AAL due to heavy grazing pressure and lower soil moisture2 probably caused significant difference in the density and biomass of earthworm between AAL and DFL. In the present study the numerical abundance of earthworms in OF is lower compared to similar land use type from Kumoan Himalayas. Besides variation in ecological characteristics probably the land use history of the ecosystems contributes to this variation, the sub-temperate climate in the study area and the relatively lower inputs of organic matter apparently provide poor conditions for earthworm populations to flourish. As a result total numbers were very low ranging from 8 to 348 m-2 as compared to high ranging from 250 to 2400 m-2 in Northern Kwazulu Natal South Africa.19 Earthworm biomass showed broadly similar trends with land use to those for abundance.
Interactions between species and sensitivity to ecological factors, presence or absence of and changes in ground vegetation composition are known to affect the composition of earthworm communities through changes in the distribution and the quality of litter, soil climate, and water availability. The presence of litter layer and lower perturbation pressure probably explains the numerical dominance of L. pussilus in the OF and the higher biomass of A. alexandri could be due to the larger size of the earthworm. B. parvus, L. pussilus and P. excavatus are litter-associated taxa which were more directly affected by OF clearance and the resulting decrease in available litter, thus explaining their disappearance in the changed ecosystems, however improved soil moisture and temperature as well as input of organic matter in RAL could probably be favorable factor for decolonization and dominance of L. pussilus. A. alexandri has wider ecological amplitude occurring under all land use types. Major determinants of earthworm communities’ structure in an agro ecosystem are the quantity and quality of organic matter added, soil type and the perturbation pressure.29 With better adaptation and tolerance to various disturbances during agro forestry practices. D. nepalensis was confined only to agroecosystems and was numerically dominant during cropping in AL (WT), in AL (T) and also in AAL. M. anamola was more directly affected due to perturbation pressure and soil degradation caused through conversion of forest to agro ecosystem,. The increased population density of A. alexandri, D. nepalensis and M. anamola in AL (T) as compared to AL(WT) is likely due to amelioration of the surface soil temperature and moisture by litter, and tree leaf biomass incorporated into the soil. The lower density and biomass of D. nepalensis under AL(WT) as compared to AL(T) could be the result of perturbations caused to the soil due to agricultural practices besides here the decline in D. nepalensis abundance in AAL and its subsequent increase in RAL could probably be because of lower soil bulk density and higher carbon percentage here and also because this land use categories maintain a year round canopy and litter layer.2 The lower biomass of A. alexandri in RFL may be due to species specific competition between M. anamola and A. alexandri as both occupy the same niche. Most changes in the distribution of earthworm species are explained by microclimate variations in soils thus low soil water content, high soil temperature, and incident radiation probably resulted in the decline in abundance and biomass of M. birmanica. in AAL and DFL as has also been shown through the studies of30 in the land use systems in eastern Zambia however improvement of soil conditions through the rehabilitation of forested land led to recurrence of this species. The traditional farming practices under rain fed conditions probably favored the population of O. beatrix species and thus explains its presence in agro ecosystem only.
Our results show that the AAL remains closer to the AL (T) than to the forest, because in these land use types the overall vegetation diversity remains low corresponding to a low diversification of the organic resources thus explaining the similarity between the communities in land use types.31 The shift in plant composition in RAL resulted in a shift in organic input from a below ground pattern in AAL to an above and below ground input here. This increase in above ground litter input created habitat in the plantation floor similar to that of OF and this probably was the reason for community structure of RAL to similar to the OF as has also been shown through the studies done by workers32 who suggested that the community structure under tree plantations is typically similar to that under native forest. The rehabilitation of DFL only caused an increase in the abundance of earthworm species in RFL resulting in community structure of RFL and DFL being closer to one another.
The size and species composition of earthworm communities are important because any shifts in earthworm community may result in significant changes in soil properties, epigeic earthworms, which usually inhabit upper soil and litter layers, may be more exposed than endogeic and anecic species that live primarily in subsurface soil. The conversion of OF to agro ecosystems led to a shift in functional categories from epigeic dominated species community to endogiec and anecic dominated composition, as has also been shown through studies of33 where the loss of the surface litter layer when rain forest is converted to agricultural use resulted in a dramatic decrease in the number of epigeic and anecic species and increase in numbers by endogeics species. The epigeics are litter-associated and thus were more directly affected by forest clearance and the resulting decrease in available litter leading to their loss in all the experimental plots except in RAL where with the deposition of litter the epiges recurred. P. excavates and B. parvus species cannot therefore survive in areas with less plant cover and litter availability and thus can be said to be bioindicator of land use that leads to these conditions. Anecic species need plant litter and specific microclimatic conditions in the soil thus the cropping activities with the removal of litter layer in AL (WT) prior to rice crop plantation resulted in decline of the anecics here. The increase in above ground litter input in AL (T) created microhabitat in the below canopy promoting the colonization of both endoges and anecic species. Further anecics are generally sparse in tropical environments,34 the experimental studies in the rehabilitated plots by Butt KR et al.35 have shown anecics to be slow colonizers in the reclaimed sites and this could probably explain their lower density in the RAL and RFL. Endogeics being geophagus, their predominance in the AL (T), AL (WT) AAL and RAL, could be because probably these habitats offered favorable base resource to the species which migrated from the surrounding plots to colonize and establish themselves in the new habitats.36,37 Dominance of endogeics in the disturbed habitats has also been shown through the studies of.38,39 As a consequence, while epigeics does not significantly alter the soil surface, casts produced by endogeics highly modify soil surface properties.
As reported in our studies conversion of OF to other land use types resulted in lower species diversity, similar result were also obtained through the studies of40 where conversion of forests to pastures resulted in decline of earthworm species diversity. Rehabilitation of these ecosystems in RAL and RFL resulted in improved diversity index of the community structure, this increase in earthworm diversity could probably be related to a shift in organic input from a below ground pattern in AAL and DFL to an above and below ground input in RAL and RFL.41 The lower overall vegetation diversity in AAL probably corresponded to a very low diversification of the organic resources and this can explain a lower diversity of earthworms here as low resource diversity leads to impoverished species diversity.38
Seasonal variation has been shown for macro faunal assemblages for temperate,42 for tropical12 and for semiarid region,43 similar seasonal rhythm for all the species was also observed in our studies. Earthworm exhibit temporal variability, population density and biomass as well as the average depth of an organisms’ position in the soil profile is greatly affected by soil temperature and humidity.44,45 such seasonal rhythms of soil macro fauna were clearly expressed in the experimental land use type. Large differences in the number of earthworms were found in the different seasons highest catches were in monsoon, with lower numbers in winter and a large reduction in summer. Many soil organisms display strong seasonality in their life cycles,46 Few earthworms were found in summer compared to monsoon months , suggesting that all species hatch during early monsoon grow to adulthood over the monsoon period and decline in winter months, the lower mean catches in summer can be attributed to a response to drought by some species, the increase in the population density and the biomass of all earthworm species during the rainy season could be attributed to better soil moisture and temperature condition which favored the growth and maturity of earthworms.47 Seasonal temperature variations commonly induce vertical movements of earthworms in as soil profile48,49 as was also observed in D. nepalensis in the AL(T) which retreated to deeper soil50 to escape lower temperature and draught by diapausing during winter.
This study has shown that earthworm communities are directly affected by alteration of land use patterns. The numbers of species present at our sites ranged between 2 to 5. All the ecological categories such as endemic and exotics co-existed in the study area following deforestation and intensive cultivation, and the disturbance and degradations led to invasion by the exotic species and the native species dominated the undisturbed sites. The sites under study represented the degraded areas and none of the species reported from the present experimental plots were endemic to the region, all the species are either peregrine exotic or peregrine endemic to the area, as many of the endemic species of this region probably exterminated during the last Quaternary Glaciation. The presence of litter layer and lower perturbation pressure resulted in the numerical dominance of epigeic L. pussilus in the Oak forest (OF) and the higher biomass of A. alexandri may be because of the larger size of the earthworm. Litter-associated taxa (Epigeics) such as B. parvus, L. pussilus and P. excavatus are more directly affected by primary OF clearance and the resulting decrease in available litter, thus explaining their disappearance in the changed ecosystems. However improved soil moisture and temperature as well as input of organic matter in rehabilitated agricultural land (RAL) were probably one of the favorable factor for decolonization and dominance of epigeic L. pussilus. Endogeic species probably are not much affected by deforestation and degradation process and therefore A. alexandri had a wider ecological amplitude occurring under all land use types, however conversion of primary OF to other land use types resulted in lower species diversity. Our results show that the earthworm diversity in abandoned agriculture land (AAL) remains closer to the AL(T) than to the forest because in these land use types the overall vegetation diversity remains low corresponding to a low diversification of the organic resources thus explaining the similarity between the earthworm communities in land use types. All the species identified at study sites showed seasonal rhythmic pattern.
The research was financially supported by TSBF-SARNET programme. Thanks are due to Dr JM Julka (Emeritus Scientist) of Zoological Survey of India, Govt. of India for taxonomic identification of the earthworm species. The authors acknowledge the help extended by the co-ordination unit based at JNU New Delhi for providing the necessary literature.
There is no financial or any conflict of interest exists.

©2018 Bhadauria, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.