eISSN: 2473-0815


Research Article Volume 10 Issue 1
Professor, College of Medicine, University of Science Arts and Technology, UK
Correspondence: Orien L Tulp, Professor, College of Medicine, University of Science Arts and Technology, Montserrat and the College of Medicine, University of Health and Humanities,Virgin Islands UK
Received: June 29, 2022 | Published: July 15, 2022
Citation: Tulp OL. Effect of dietary carbohydrate type, glycemic index, and NIDDM on brown adipose tissue hypertophy and function in the SHR/Ntul//-cp RAT. Endocrinol Metab Int J. 2022;10(1):20-27 DOI: 10.15406/emij.2022.10.00314
To determine the effects of carbohydrate (CHO) type and NIDDM on the characteristics of interscapular brown adipose tissue (IBAT) growth and development, and on thermogenic responses to diet and environment, groups of lean and obese-NIDDM SHR/Ntul//-cp rats were fed nutritionally complete isoenergetic diets containing 54% CHO as cooked cornstarch (low glycemic index, LGI diet) or the same diets plus substituting Sucrose for cornstarch (S, high glycemic index, HGI diet) for up to 9 months of age. Measures of blood Glucose, Insulin and the Insulin to Glucose (I:G) ratio were increased in the obese+NIDDM phenotype and further compromised in obese-NIDDM rats by HGI diet. Measures of RMR in vivo were decreased in the obese-NIDDM but increased in both lean and obese+NIDDM rats following norepinephrine administration (100 µg/kg BW, s.c.) phenotype, while isoenergetic substitution of sucrose vs cornstarch in subgroups of each phenotype resulted in modest increases in both phenotypes. Measures of urinary vanilmandelic acid (VMA) excretion and temperature regulation following 4°C cold exposure as measures of in vivo sympathetic activity were decreased in the obese+NIDDM phenotype but improved modestly with the isoenergetic HGI regimen in both phenotypes. At 9 months of age, both the white adipose tissue (WAT) and IBAT mass and cellularity were markedly greater in obese+NIDDM than lean animals. These results indicate that despite increased VO2 following exogenous administration of NE and a diet effect on VMA excretion in both phenotypes, the in vivo capacity for expression of NST is qualitatively decreased in obese+NIDDM rats, and the greater glycemic index and resulting increase in parameters of insulin resistance may be contributory factors in the magnitude of adiposity regardless of the dietary carbohydrate source.
NIDDM, non-insulin-dependent diabetes mellitus; SHR, spontaneously hypertensive rat; OsO4, osmium tetroxide; VMA, vanilmandelic acid; PEM, protein energy malnutrition; IBAT, interscapular brown adipose tissue; NST, nonshivering thermogenesis; SNS, sympathetic nervous system; IACUC, institutional animal care and use committee
Brown adipose tissue (BAT) has long been known to play a fundamental role of heat production in mammalian organisms, and has been identified in man and animals both histologically and metabolically for several decades.1–5 Although the earliest observations of brown adipose tissue by anatomists date to the 16th century, only in recent decades have the biochemical and physiological roles of BAT in energy homeostasis been elucidated, and its potential therapeutic contributions to whole body energy regulation proposed.6–9 BAT is the only known tissue with a primary function of heat generation as a mechanism of energy expenditure in response to changes in diet and environment. While its role in heat production among the newborn and in hibernating animals of several species is well established, the contributions of BAT-mediated contributions to mechanisms of energy balance in adult humans remains unclear.10–14 Tanuma et al documented the presence of BAT in autopsy findings throughout much of the human lifespan, and Tulp et all documented the presence of BAT in lean and obese rats throughout much of their lifespan in rats as well.7,15,16 The contributions of brown adipose tissue to parameters of non-shivering thermogenesis in response to perturbations in diet and environment in man and animals has long been established where it requires contributions from insulin, norepinephrine, and other biochemical factors likely including batokines to facilitate interorgan and multi-tissue communication and to augment the expression of thermogenic activity and energy homeostasis.17–20 Sensitivity to Insulin in isolated brown adipocytes in vitro has been reported to be an essential element in the expression of thermogenic activity, but in vivo studies in intact animals exhibiting significant insulin resistance typical of NIDDM have seldom been reported. The SHR/Ntul//-cp rat is an animal model of obesity+NIDDM where resting VO2 becomes significantly decreased early in life, while the cellularity and physiologic functionality of BAT mediated thermogenesis in obese+NIDDM animals has not been fully described.21–23 High glycemic index dietary carbohydrate sources including sucrose enriched diet regimens can bring about increases in thermogenesis in man and animals, with the simple carbohydrate sources such as glucose or sucrose being among the most effective in normally lean and otherwise healthy animals. In those studies one or more aspects of thermogenesis were found to become impaired among obese rats fed HGI dietary regimens where the caloric efficiency of weight gain was also more favorable than was observed in their lean littermates and increases in adiposity prevailed.14,15,21–23
The isoenergetic substitution of sucrose for cornstarch was found to result in greater plasma insulin and triglyceride concentrations and in greater weight gain in obese than in lean rats, consistent with the development of other markers of insulin resistance among the obese but not among similarly fed and reared lean non-diabetic rats.24–26 The long-term feeding of the sucrose- enriched diets has also been shown to result in a decreased capacity for nonshivering thermogenesis (NST) in both lean and obese phenotypes, and where BAT is presumed to have been a principal tissue contributing to the expression of the thermogenic responses. Insulin contributes to a key hormonal function in the expression of NST where in conditions of normal insulin sensitivity it exhibits a permissive action in part due to its role in modulating glucose uptake in multiple peripheral tissues including BAT. However, Marette et al demonstrated that under conditions of insulin resistance the thermogenic responses to norepinephrine in isolated brown adipocytes in vitro were impaired suggesting that insulin resistance may be a significant contributing factor in the impaired thermogenesis and thermoregulation observed in obese and obese-NIDDM rats.17,19,20
Cold exposure is a well-established and documented process to bring about increases in thermogenesis in mammalian species, via combinations of shivering in the initial physiologic phases followed by activation of non-shivering metabolic processes as the duration of cold exposure progresses. Both thermogenic mechanisms, albeit it by different but complimentary biochemical and physiologic processes bring about the generation of heat in an apparent attempt by the organism to maintain viable body temperatures necessary for survival.1-3,27–29 Prolonged cold exposure in obese animals often results in greater decreases in body temperature than occurs in lean animals of the same strain, consistent with a diminished capacity to maintain homeothermy.27–30 Dietary composition also contributes to the non-shivering elements of thermogenesis, ostensibly as an assist to balance essential nutritional requirements with energy needs, and which can enable an animal to dispense with excess non-essential energy components via dissipation of the excess calories in the form of heat of oxidation, and easily quantified via measures of VO2 in the resting or metabolically perturbed state. Among the most readily oxidized macronutrient sources are simple carbohydrate sources, including mono-and disaccharides which when metabolized typically yield an RQ of approximately 4.0. Thus, although not the most energy dense of the common macronutrient sources, the simple CHOs are readily oxidized by virtually all known metabolically active tissues and thus can lend to more rapid but less sustained energy release over longer durations than proteins or fats. Complex CHO sources are readily degraded in their entirety via a complex of glucosidase enzymes to yield simple monosaccharides in the gut, where they are efficiently absorbed into the peripheral circulation with little or no net loss of metabolizable energy. Simple CHOs including glucose, sucrose and related sugars typically yield the highest glycemic index approaching 1, associated with greater increases in initial insulin requirements, while complex CHOs including cornstarch and fructans typically take longer to digest in the luminal epithelium, delays in luminal absorption, and a proportionately lower glycemic index.31
As summarized above, the defined role of brown adipose tissue (BAT) and the sympathetic nervous system (SNS) activity in the expression and development of mechanisms of non-shivering thermogenesis in rodents and other mammals is well established. Because the primary function of BAT is deemed to convert energy derived from food or stored lipid into heat via intracellular oxidation and with the further dissipation in the form of heat energy, the thermogenic actions of BAT have been proposed to contribute to mechanisms of energy balance, and when impaired to be a potential contributor to the expression and development of obesity in man and animals.1,2 BAT is especially important in the newborn of several mammalian species including humans, and in maintaining body temperature during the early hours following birth and following acute cold exposure and in chronically cold environments in younger and older animals.1–5,30,32
BAT normally becomes activated via initial neurohormonal signals initiated from the hypothalamus, and transmitted to the BAT directly via sympathetic neurons, which bring about the release of the neurohormone norepinephrine, a β-adrenenoreceptor agonist.1–5 BAT tissues contain specialized stereospecific β3-adrenergic receptors, unique to brown adipocytes which bring about the activation of the thermogenic activity. The thermogenic response occurs following the hydrolysis of high energy phosphate bonds, each of which can release approximately 7.2 kcals of energy in the form of heat.15 The uncoupling protein-1 is unique to BAT (UCP1) and mediates the activation of thermogenesis in mammalian species via a stereospecific action, while UCP analogues UCP2 and UCP3 were ineffective.1,2,33 Himms-Hagen, Rothwell and Stock, and others have established the role and biochemical mechanisms of BAT-mediated thermogenesis in rats and humans, and Tulp et all established the effects of early overnutrition and of nutritional manipulation on hyperplasia and hypertrophy of brown adipose tissue in young Sprague-Dawley rats when overfed or fed calorically adequate but protein restricted diets from weaning to adolescence.1–5 Once formed, the increased BAT mass and cellularity persisted well into adulthood in those studies.15,16 In those early studies, the BAT mass and cellularity increased by more than 2-fold during the early postweaning period. The thermogenic responses to norepinephrine occurred normally in proportion to the increased BAT mass in metabolically normal lean rats, but catecholamine-stimulated responses in obese and obese diabetic animals demonstrating various degrees of insulin resistance have been shown to become impaired by early adulthood. Marette et al. demonstrated that insulin sensitivity Is an essential element in peripheral glucose uptake and in the catecholamine mediated expression of BAT thermogenesis and Tulp et all showed that the resting, and norepinephrine stimulated thermogenesis in obese diabetic (NIDDM) rats of several strains demonstrated impaired thermogenic responses to diet and environment. 9–11,15,16,19,22,24,28,34
The peripheral uptake of glucose, an insulin dependent process in muscle and adipose tissue has also been linked to the expression of thermogenesis in peripheral tissues.17,19,20 Himms-Hagen and others have suggested that a fundamental role of BAT thermogenesis in the early life of a mammalian species is to generate necessary heat ostensibly to contribute to temperature regulation in the newborn and early life stages, where the surface area to metabolic mass is greater in proportion than that which occurs later in life including adulthood.1–3,9–11 In addition, the added benefit of extra BAT activity could also facilitate biochemical mechanisms that contribute to energy balance by converting excess energy to heat, thereby minimizing the effects of the excess caloric impact on fat accretion in adipose tissue. The development of the obese (-cp) phenotype occurs via an epigenetic expression, commencing soon after weaning in genetically obese rodents.35The cellular characterization of brown adipose tissue in lean animals has been described but the BAT characteristics in obese NIDDM rats of this strain have not heretofore been described. The early and chronic hyperphagia common among obese and obese NIDDM strains in concert with enhanced caloric efficiency are suggestive entities as contributing predisposing factors in induction of the hypertrophy and hyperplasia of both WAT and BAT depots. This may occur as a physiologic attempt by the animal to attenuate the potentially adverse impact of the early onset hyperphagia on development of comorbidities via enhanced energy deposition and storage as fat, which should it become extreme may result in dire outcomes with regards to health and longevity in man and animals.12 ,15,30
The activation of nonshivering thermogenesis in the obese-diabetic (NIDDM) SHR/Ntul//-cp rat has been shown to be impaired, at least in part secondary to the effects of insulin resistance in brown adipose tissue (BAT), where sensitivity to insulin actions is essential for expression of thermogenesis in isolated brown adipocytes.17–20 Impaired parameters of thermoregulation and energy expenditure facilitates an efficiency and ease of weight gain, contributing to the epigenetic expression, development, and maintenance of an obese phenotype.35 The magnitude of insulin resistance is further magnified in the presence of NIDDM, which likely further impairs unobstructed thermogenic activity. BAT is abundantly innervated by the sympathetic division of the autonomic nervous system and is increased in lean animals during periods of caloric overnutrition, including carbohydrate feeding.1–3 As a result of the increased SNS activity in response to diet and environment, urinary excretion of norepinephrine metabolites via conversion to vanilmandelic acid becomes increased in proportion to the SNS activity and appears in the urine.36 Glycosuria can contribute to an osmotic diuresis, resulting in increased volumes of urine especially in uncontrolled NIDDM, while the osmotic impact of the urinary catecholamine metabolites is relatively minor in nature owing to the smaller contributions to osmolality and final urine volumes but is nonetheless reflective of the magnitude of SNS activity.34–36
The incidence of overweight and obese conditions has now become prevalent in numerous developed societies. This development has occurred in concert with advances in nutritional science and technology, but where the incidence of overweight and obese conditions now borders on becoming epidemic, in spite of numerous advances in medical knowledge and practice.32,37,38 The overall impact on society of obesity and its related comorbidities including NIDDM, cardiovascular disease, and others has now placed an enormous burden on the economy due to lost productivity, and on the health care resources of developed societies.
The development of the SHR/N-cp rat strain is schematically depicted in Figure 1 below and shows that the -cp (corpulent) trait originally derived from the Koletsky rat was bred into the Spontaneously Hypertensive Rat (SHR) rat at the Veterinary Resources Branch of the NIH by Hansen as part of a then ongoing project to develop a congenic and SPF animal model for investigation of obesity and its comorbidities.21,37 The current SHR/Ntul//-cp rat strain was established from original breeding pairs derived from the 12th backcross of the original stock. The -cp trait is transmitted as an autosomal recessive trait now known to be located on chromosome 5 and becomes evident in one quarter of the offspring of heterozygous breeding pairs by 6 weeks of age, and early stigmata of NIDDM appears spontaneously by 8 to 10 weeks of age among the obese offspring.15,40
Groups of 4–5-week-old male lean and obese littermates of SHR/Ntul-cp rats were obtained from the Drexel colony, originally developed from breeding pairs obtained from the Veterinary Resources Branch of the NIH in Bethesda, MD, USA. Rats were fed a standard diet consisting of 54% CHO as cornstarch, 22% protein, 16.5 % mixed fats, 4.5% crude fiber plus essential vitamins and minerals from four weeks until 9 months of age. Animals were housed in their prechilled hanging steel cages at 22 °C and 50% RH on a reverse light cycle. Measures of body weight were obtained periodically throughout the study. Rats were sacrificed by decapitation, fasting bloods collected for measures of glucose and insulin concentration, and the Interscapular (IBAT), Dorsal, Retroperitoneal and Epididymal fat depots dissected in their entirety. Urine was collected in 1 ml of 6N HCL to preserve catecholamine metabolites over 24-hour periods and urine volumes recorded.18 Sections of IBAT of approximately 50 mg each were weighed to the nearest 0.005 g, and double-fixed with formalin and osmium tetroxide (OsO4) as described by Tulp et al and the particle size and volume determined on a Coulter particle counter.4,12 In WAT depots, 50 mg sections of tissue were collected and fixed directly in OsO4 solution to fix and immobilize the lipid content, and counted in a Coulter particle counter.4 Tissue lipid content was determined gravimetrically as described by Hirsch and Gallian and tissue protein content as described by the Lowry phenol procedure.40,41 Urine glucose was determined qualitatively, and urinary vanilmandelic acid as a reflection of sympathetic activity measured as outlined previously.22,36 Tissue depots were dissected in their entirety and weighed on an Ohaus electronic balance to the nearest 0.1 gram within one minute of dissection, and before significant dehydration could occur. Values beyond 9 months of age were not obtained in this or other studies with this strain as the lifespan of the obese-NIDDM rats began to decline beyond 10 months of age and seldom exceeded 12 months of age, while the lean phenotype was often observed to survive to 12-15 months of age. Measures of resting and norepinephrine-stimulated oxygen consumption were determined via closed circuit thermography with a Collins small animal thermogenesis apparatus at thermal neutrality as described previously.15,26 Measures of rectal and colonic temperature were determined with a petrolatum-lubricated YSI thermistor at retroanal distances of 3 and 12 cm in quietly resting animals as indicators of thermoregulation before and following 4 hours of 4°C cold exposure individually in their hanging steel cages.28 Data were analyzed via standard statistical procedures including Students t test and ANOVA. The study was approved by the institutional animal care and use committee (IACUC).
In the present study, groups of congenic lean and obese male SHR/N-cp rats as biological littermates were maintained in hanging wire-bottomed steel cages and fed a nutritionally complete diet containing 54 % CHO, 22% protein,16.5 % mixed fats, and 4.5% essential fiber, plus vitamins, minerals, and essential micronutrients from 1 to 9 months of age. Measures of body weight were monitored and 24-hour urinary vanilmandelic acid (VMA) were determined at the end of the study. Animals were sacrificed by decapitation and the Interscapular BAT depots excised in their entirety for measures of adipocyte size, number, and lipid content. Body weights, net weight gain and relative adiposity of Obese was significantly greater than their lean littermates throughout the study. Urinary VMA of lean > obese rats. The IBAT weight and IBAT weight: Body weight of obese >> lean. IBAT cell number, cell lipid content and % lipid of IBAT tissues of obese >> lean of obese rats. The results of this study indicate that while the development of IBAT mass and cellularity becomes exaggerated in the obese-diabetic animals, the superimposition of the NIDDM stigmata including likely insulin resistance may further compromise the capacity of the obese diabetic animals to fully express BAT-mediated contributions to NST.
The initial and final body weights of lean and obese animals are depicted in Table 1 & Figure 2, and clearly show that the obese rats while similar in body weight at the onset, gained considerably more weight and were nearly twice as heavy as their lean littermates by 9 months of age. The sucrose diet was associated with modest additional increments in final body weight and net weight gain. The net gain is depicted in far-right column of each panel and depict the net gain of each phenotype and diet group. The phenotype effects on body weight and weight gain were highly significant by Students t test. The fasting glucose, insulin, and insulin to glucose ratios are depicted in Figure 3 and indicate that fasting glucose and insulin concentrations were well within normal ranges in the lean phenotype but were significantly greater in the obese phenotype. The differences in the Insulin to glucose ratio were also markedly elevated in the obese-NIDDM phenotype, consistent with significant insulin resistance among the obese phenotype of this strain and which were further increased in the rats consuming the HGI sucrose regimen. As noted above, values beyond 9 months of age were not obtained in this or other studies with this strain as the health and lifespan of the obese-NIDDM rats was observed to deteriorate prior to 12 months of age.
Group |
N |
Initial Weight, g |
Final Weight, g |
Net Gain, g |
Glycosuria |
Lean |
6 |
63±4 |
455±6 |
392±8 |
no |
Lean + S |
6 |
65±3 |
471±8 |
406±11 |
no |
Obese-NIDDM |
6 |
69±5 |
820±32 |
752±21 |
yes (4+) |
Obese-NIDDM+S |
6 |
70±5 |
865±21 |
796±26 |
yes (4+) |
ANOVVA |
p = |
NS |
< 0.05 |
< 0.05 |
< 0.05 |
Table 1 Body weights, weight gain and glycosuria of rats
Data are mean±1 SEM, n= 6 rats/treatment group.

Figure 2 Effect of diet and phenoyupe on body weight of rats.
Data are mean ±1 SEM, n = 6 rats per treatment group.
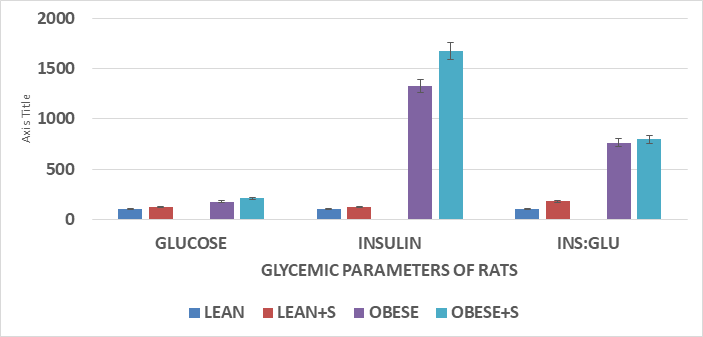
Figure 3 Glycemic parameters of rats.
Data are mean ±1 SEM, n= 6 rats / phenotype group. Glucose presented as mg/dl; Insulin as µU/ml and the Insulin to glucose ratio computed and expressed by dividing insulin concentration in µU/ml by the glucose concentration in mg/dl. X 100.
The results of phenotype on cellularity and relative adiposity of multiple white adipose tissue depots are shown in Table 2 and of IBAT parameters in Table 3, and indicate that the mass of combined white adipose tissue depots and the relative adiposity of obese was significantly greater than was observed in the lean phenotype but were not significantly impacted by diet. The hyperplasia and hypertrophy of the WAT is reflective of the greater WAT mass, in that both adipocyte number and adipocyte size averaged an approximate 2-fold increase over their similarly fed and reared lean littermates. The effects of phenotype on the mass and cellularity of the interscapular brown adipose tissue (IBAT) are shown in Table 2 & Figure 4 and indicate that the mass of the IBAT of the obese-NIDDM rats greatly exceeded the IBAT mass of their lean littermates. Of interest, the usual deep brownish coloration of the IBAT of the obese-NIDDM rats was less intense than occurred in their lean littermates, consistent with the greater percent lipid content and lower protein content in those animals. The adipose cellularity of obese-NIDDM rats greatly exceeded the cell number per depot in the lean littermates, suggestive at least in part that the early hyperphagia common to the obese phenotypes of this and other strains of obese rats may have contributed to the IBAT hyperplasia. IBAT cell size as determined by cell lipid content was also greater in the obese than the lean phenotype and is likely suggestive of diminished endogenous thermogenic activity under thermal neutral conditions. In earlier studies performed early in the course of development of NIDDM, the resting metabolic rates were observed to be 17% lower in obese than in the lean littermates, while both phenotypes responded to exogenously administered norepinephrine.22
Group |
N |
WAT Depot, g |
WAT:BW |
WAT Cells x 106 |
WAT Cell lipid, nl. |
Lean |
6 |
17.5±0.4 |
3.85±0.05 |
43±3 |
0.36±0.07 |
Lean +S |
6 |
20.2±0.3 |
4.29±0.06 |
45±5 |
0.37±0.06 |
Obese-NIDDM |
6 |
137.9±2.3 |
16.82±0.50 |
94±7 |
0.57±10 |
Obese-NIDDM+S |
6 |
143.4±2.9 |
16.64±0.60 |
86±6 |
0.62±0.15 |
Phenotype |
P = |
<0.01 |
<0.01 |
<0.01 |
<0.05 |
Diet |
p = |
n.s. |
n.s. |
n.s. |
n.s. |
Interaction |
p = |
n.s. |
n.s. |
n.s. |
n.s. |
Table 2 White adipose tissue cellularity of SHR/Ntul//-cp rats
Data are mean±1 SEM, n=6 rats/treatment group. Depot weights and Cell number = combined sum of epididymal,
retroperitoneal and dorsal adipose tissue depots.
Group |
N |
mg/IBAT depot |
% Lipid |
Mg Protein/depot |
Cells X 106 |
Cell size, µg |
Lean |
6 |
650±33 |
52±2 |
130±3.3 |
2.4±0.1 |
0.23±0.06 |
Obese-T2DM |
6 |
5930±200 |
77±3 |
110±4.7 |
11.7±2.7 |
0.86±-0.12 |
P= |
<0.001 |
<0.01 |
<0.05 |
<0.05 |
<0.05_____ |
Table 3 Tissue mass, composition and cellularity of IBAT depots in SHR/Ntul//-cp rats
Data are mean±1 SEM, n=6 rats/treatment group. IBAT lipid. Cell lipid content expressed as µg lipid per cell. Determination of values in the Fructose group were not determined.

Figure 4A IBAT Parameters of rats. IBAT mass (g/depot), IBAT:BW (percent of BW), IBAT cellularity (x 106/depot), and adipocyte size (µg lipid/cell).

Figure 4B Composition of IBAT in lean and obese rats.
Data are mean ±1 SEM, n= 6 rats/group. Grams lipid or protein were computed by multiplying the percent in mg x the weight of the depot in mg.
The results of phenotype on glycosuria and daily (24 hour) vanilmandelic acid (VMA) excretion are depicted in Figure 5 and indicate that the urine qualitatively reflected significant glycosuria in the obese NIDDM compared to the lean non-diabetic animals. In contrast, urinary VMA excretion of lean animals was significantly greater than was observed in the obese phenotype, indicative of less endogenous sympathetic (SNS) activity among obese-NIDDM animals, despite having a greater mass and cellularity of IBAT but indicative of lesser magnitude of SNS activity.
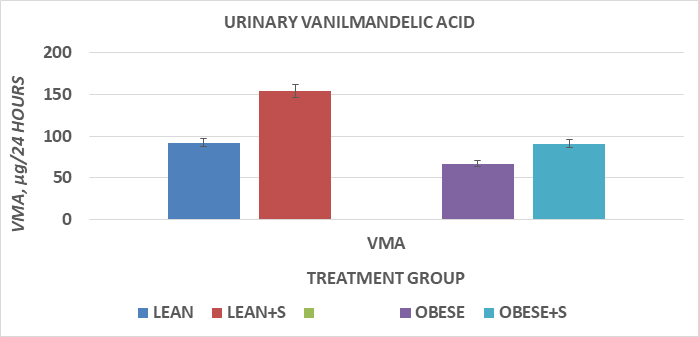
Figure 5 Daily Vanilmandelic acid (VMA) excretion expressed in µg/24 hours.
Glycosuria (4+) was present in all obese NIDDM rats, but not in lean rats. Data are mean ±1 SEM, n=6 rats / group. Both phenotype and diet were significant at P= <0.05, where Lean > Obese and Sucrose > cornstarch as CHO in both lean and obese rats.
Measures of thermoregulation are depicted in Figures 5 thru 7 and indicate that VMA excretion was greater in lean than in obese+NIDDM rats, and was greater in both phenotypes when fed the HGI sucrose dietary regimen, but the magnitude of sucrose induced increase of obese+NIDDM rats was significantly less than occurred in their lean littermates fed the same dietary regimen (Figure 5). In Figure 6, the measures of VO2 in the resting and NE-stimulated state are depicted, and show that consistent with earlier studies, the RMR of lean rats was greater than occurred in their lean littermates. In addition, the sucrose dietary regimen was associated with only a modest increasing trend in RMR in both phenotypes. Injection of a submaximal dose of norepinephrine, a potent β-agonist with β3-adrenoreceptor activity resulted in significant increases in VO2 in both phenotypes, with the quantitatively greatest increments in the lean+S group. The effects of cold exposure in a prechilled (4°C) cold environment resulted in decreases in both rectal and colonic temperature in both phenotypes, with the greatest decrease observed among the obese+NIDDM rats. Of interest, the HGI-sucrose dietary regimen occurred without a significant protective effect on maintaining thermoregulation in either phenotype, consistent with the modest impact observed on RMR and following administration of a submaximal dose of NE in the obese phenotype.
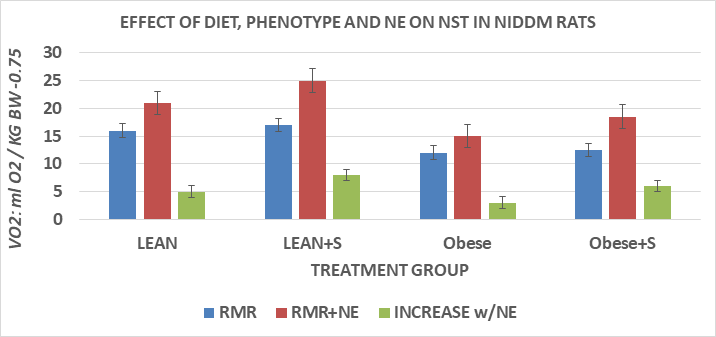
Figure 6Effect of diet, phenotype and norepinephrine (NE) on non-shivering thermogenesis in NIDDM rats.
Data are mean ±1 SEM, m=6 rats/group. RMR obtained in fasting animals; NE administered subcutaneously at dose of 100 µg/kg BW 15 minutes prior to recording. All measures determined at thermal neutrality for rats (30°C) and expressed as ml O2/kg BW/minute-.0.75 as described by Kleiber and Yang et al. to correct for differences in body surface area.42,43
This study represents the first definitive study on adipose cellularity and capacity for thermoregulation of the brown adipose tissue in the SHR/Ntul//-cp NIDDM rat. BAT is normally a major contributing tissue of non-shivering thermogenesis in rodents, and it has been shown to become increased in both mass and cellularity in healthy lean non-diabetic animals following dietary intervention with HGI dietary regimens. In addition the BAT also exhibits greater thermogenic activity during dietary overnutrition, cold exposure and following the feeding of calorically adequate but protein restricted diets otherwise typical of protein energy malnutrition (PEM) dietary regimens in lean rats.16,44 In the present study, all animals received a nutritionally adequate diet, ad libitum from 4 weeks until 9 months of age, thereby representing much of the typical lifespan of this strain of rat.15,27 The IBAT development occurred normally as predicted in the lean phenotype, but the IBAT depot mass and cellularity of the obese-NIDDM rats were significantly increased by processes of both hyperplasia and hypertrophy. While increases in BAT mass and cellularity would normally be expected to result in corresponding increases in sympathetic activity and thermogenic capacity, in addition to proportional increases in urinary VMA excretion and in an improved capacity for thermoregulation, the greater mass and cellularity of the obese-NIDDM phenotype was associated with a lesser magnitude of sympathetic activity that would have been expected in the absence of obese-NIDDM stigmata. Consumption of a HGI-sucrose dietary regimen was associated with greater VMA excretion in lean rats, but the VMA and thermogenic responses in obese+NIDDM rats were less than proportional to the greater IBAT mass and cellularity observed. These results are thus indicative of decreased SNS activity and a decreased endogenous capacity for NST in the obese+NIDDM phenotype. In previous studies, the obese NIDDM phenotype was found to exhibit an impaired capacity to maintain core body temperature when exposed to a 4°C cold environment consistent with the decreased SNS activity and in the present study the magnitude of impairment in the obese+NIDDM also occurred, and the HGI-sucrose dietary regimen provided little if any thermogenic protection. That resulted in lesser rates of VMA excretion than occurred in lean animals of the present study.22
The extent to which the decreased SNS activity at 9 months of age may have contributed to the net fat accretion in adipose depots is speculative but is likely a contributing metabolic factor along with the hyperinsulinemia also observed. Insulin exerts numerous hormonally linked biochemical effects in peripheral tissues where it is widely regarded as a lipogenic hormone capable of promoting both de novo lipid biosynthesis in liver and adipose tissue in addition to impaired lipid mobilization in adipose tissues.9,10 The deposition of both dietary and de novo formed lipids are readily deposited into adipose tissue with a minimum of energy expenditure, therefor further enhancing the caloric efficiency of the obese-NIDDM phenotype. Thus, the mass and cellularity of BAT is not by itself a reliable marker of endogenous thermogenic activity, but may reflect dietary, environmental, genetic and age-related influences in its development and capacity for thermogenic activity. In the earlier studies, the RMR of 3 months-old obese-NIDDM rats was observed to be significantly lower than was observed in their lean littermates, similar to the present study while exogenous norepinephrine administration at a submaximal subcutaneous dose of 100 µg/Kg BW resulted in increases in NST of similar magnitude in both phenotypes at that age.22 The onset of significant insulin resistance progressing to NIDDM first appears at approximately 8 to 10 weeks of age in the obese phenotype of this strain, and thus likely contributed to the impaired parameters of NST observed.21–23
The IBAT was found to have a decreased intensity of pigmentation in the obese-NIDDM rats, a greater percent lipid content and a proportionally decreased protein content when compared to the IBAT from similarly reared lean littermates. The greater proportion of lipid in the IBAT from the obese-NIDDM rats is consistent with the recent reports of the presence of beige adipose tissue in other strains of obese rats by other authors and would be expected as the normally rich brownish coloration of BAT becomes diluted with additional fat accretion and in the enlargement of lipid locule size in brown adipocytes. This also occurs with the additional infiltration of cells that are indistinguishable from white adipocytes, likely migrated from nearby and surrounding WAT depots. In BAT, the multiple lipid locules common to brown adipocytes undergo a transition in relative potential for thermogenic activity that includes volumetric lipid locular expansion of their normally small lipid deposits, while retaining their unique histologic structure of a centrally located nucleus and multiple but now larger lipid locules. The administration of β-blocker agents has also been shown to result in histologic evidence of larger lipid locules in brown adipose tissue.27,28 This observation is consistent with decreased mobilization of lipid from the multiple lipid locules normally present in brown adipocytes, and in a decrease in cellular histologic evidence of thermogenic activity of BAT tissues following the β-adrenergic blockade.27 In acute starvation, where resting metabolic rates becomes decreased the locularity of brown adipocyte lipid locules has been shown to become decreased in histologic examinations as reflected by increased BAT activity that likely contributes to thermoregulation in the absence of heat generated via dietary energy sources.44–48 In contrast, white adipocytes display a single large lipid deposit, surrounded by a thin rim of cytoplasm and a peripherally located flattened nucleus, and a cellular lipid content that often exceeds 1 µg of lipid per white adipocyte in both humans and in obese rodents. The combined metabolizable locular surface area of the multiple small diameter lipid locules of brown adipocytes far exceeds the metabolizable surface area of the single larger lipid droplet commonly present in while adipocytes, thereby enabling a more rapid mobilization of FFA for activation and support of the thermogenic process of brown adipose tissues.
Daily caloric intake and the glycemic index of dietary regimens are principal factors in determining numerous parameters of energy metabolism, including hormonal contributors such as insulin and measures of thyroidal activity which often reflect the relative contributions of the carbohydrate digestibility of the dietary regimens under study. Luminal absorption of high sucrose diet regimens carry with them a greater glycemic index and greater potential for development of increased insulin release to effect the uptake of glucose in peripheral tissues. In obesity and NIDDM a high glycemic index dietary regimen is often associated with greater degrees of insulin resistance and its associated comorbidities than would be predicted in animals fed an isoenergetic complex carbohydrate diet with a low glycemic index rating. In the absence of obesity or the obese-NIDDM phenotype, increases in daily caloric intake and in the efficiency and speed of luminal monosaccharide absorption would be predicted to result in increases in the plasma concentrations of both insulin and thyroid hormones, including T3 generated in peripheral tissues from T4. The hormonal contributions and their corresponding effects in the rates of metabolism and of VO2 generated from the peripheral tissues impacted by the activation of thyroidal activity. Gavin et al. has shown that the peripheral generation of T3 from T4 in peripheral tissues is impaired in NIDDM, secondary at least in part to the insulin resistant state commonly present in NIDDM.49–51 While measures of thyroidal activity were not quantified in the present study, other studies in obese rats bearing the same -cp trait and similar evidence of insulin resistance and impaired thyroidally- mediated actions including measures of NST were impaired in both aging and obesity.22,30,31
An environmental impact may also have occurred in the present study as the animals were housed in hanging steel cages maintained at 22°C throughout the study, likely imposing a mild thermogenic stimulus, compared to a more thermal-neutral environment of pine shaving lined shoebox cages which could provide more warmth than the steel cages where BAT tissues could be more prone to involution. The NE stimulation provided results that were modestly greater than was anticipated in the obese phenotype, indicative of at least partially active β3 adrenoreceptors in the BAT of both phenotypes. Intermittent cold exposure has been shown to result in increased BAT thermogenic activity, and thus is suggestive of BAT as a potential therapeutic mechanism to increase the capacity for energy expenditure as a potential therapeutic regimen for the treatment of obesity and NIDDM as has recently been proposed by multiple authors.33,52–60
Although daily caloric intake was not determined in the present study, the increases in IBAT mass and cellularity are consistent with the hyperphagia noted elsewhere among obese animals bearing the same epigenetic -cp trait. The characteristics of non-shivering thermogenesis have been reported to be impaired in both obese and obese-NIDDM rats bearing the common -cp trait, despite having a greater mass of BAT than occurs in their lean littermates.20,32 While measures of metabolic rate following maximal dosages of norepinephrine administration or the thermogenic impact of exogenously administered norepinephrine were not included in the current study, norepinephrine is the neurohormone that normally innervates BAT via stereospecific β3 adrenoreceptors, and the greater IBAT mass exhibited hyperplasia and hypertrophy of brown adipocytes and would normally be expected to demonstrate an increased capacity for NST independent of the stigmata of obesity and NIDDM.1-3,5 In the presence of the obese-NIDDM stigmata and its associated insulin resistance however the endogenous capacity for peripheral glucose uptake in BAT and other tissues, with the consequence that BAT-mediated NST would likely become impaired, thereby enabling a greater efficiency of caloric utilization and storage mostly in the form of triglyceride in multiple adipose tissue depots.1,2 Additionally, over the duration of this study, an increase in hyperplastic-hypertrophic adiposity and fat net accretion was reflected in principle adipose tissue depots. In the present study, the NIDDM stigmata of obese animals remained untreated, resulting in significant hyperinsulinemia, insulin resistance and excess fat accretion in principal and easily quantifiable depots. The extent to which dietary or pharmacologic control of the obese-NIDDM sequelae might facilitate a restoration of thermogenic activity and processes of disordered metabolism and caloric efficiency are unknown, and remain an interesting possibility for further study. If such measures were to be applied to affected individuals of industrialized societies one may speculate that proper and clinically effective management could improve the health, wellbeing, and economic burdens of global populations, particularly those of industrialized societies.
The authors thank Dr Carl Hansen of the VRB, NIH for the generous donation of breeding stock, and the late Dr O.E. Michaelis and Dr. Susan DeBolt of Drexel University for material contributions to this study.
The authors declare no conflicts of interest.

©2022 Tulp. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.