eISSN: 2373-6372


Research Article Volume 13 Issue 1
Department of Medicine, Colleges of Medicine and Veterinary Medicine, University of Health and Humanities Virgin Islands
Correspondence: Orien L Tulp, University of Science Arts and Technology, Montserrat, BWI, the University of Health and Humanities,Virgin Islands, Tortola BVI, and the Einstein Medical Institute, North Palm Beach, FL, USA
Received: December 18, 2021 | Published: January 28, 2022
Citation: Tulp OL, Einstein GP, Awan AR. Effect of a chromium-fructan complex on glycemic parameters in obese and obese-T2DM/NIDDM rats. Gastroenterol Hepatol Open Access. 2022;13(1):5-10. DOI: 10.15406/ghoa.2022.13.00487
The incidence and odds ratio of obesity and Type 2 diabetes, a common sequela of obesity, are increasing in incidence to the point of becoming epidemic in Western society. To determine the potential beneficial effects of a chromium-fructan complex (CFC) on glycemic responses of male obese and obese diabetic (T2DM/NIDDM) rats, groups of 3-4month old lean and obese LA/Ntul//-cp and SHR/Ntul//-cp rats (T2DM/NIDDM-rats, n= 6 rats/group) reared normally on Purina #5012 chow from the time of weaning, were subjected to an oral glucose tolerance (OGT) after a brief 4-hour fast. A second group of similarly reared T2DM/NIDDM rats (n=5) aged one year were also administered the OGT and CFC challenges. The T2DM/NIDDM rats develop IGT, elevations in plasma insulin and amylin concentrations by adolescence and glycosuria, proteinuria, renal disease, cataracts, and impaired nerve conduction velocity typical of T2DM/NIDDM by adulthood. Animals were administered a glucose solution equivalent to 2.5grams/kg BW via intragastric gavage, or the same glucose plus the CFC (2.5g/kg BW, containing 200µg Cr/serving) or the CFC alone over a brief (1-2 minute) duration. Measures of blood glucose (BG) were determined at 30-minute intervals from zero to +120 minutes post gavage via tail bleeding, and the area under the curve (AUC) of each group determined. Final body weights (BW) of obese were greater than lean (p=0.05), and obese-T2DM/NIDDM > Obese rats (p=<0.05) and increased further in T2DM/NIDDM rats with older age. OGT of lean rats remained normal throughout, and CFC resulted in a minor decreasing trend in AUC and in BG concentrations at all time points (p= n.s.) The OGT of obese rats demonstrated significant increases in BG at +60, +90-, and +120-minutes post gavage but remained non-diabetic, and CFC decreased the glycemic increase and AUC by ~50%, similar to the glycemic response off lean littermates. The OGT of young T2DM/NIDDM was significantly impaired and CFC resulted in a 32% reduction in the AUC. The OGT and AUC of OLD T2DM/NIDDM rats were greater than in young rats and were decreased by 25% with CFC. OGT and OGG+CFC were similar at both ages in NIDDM rats. These results are consistent with a CFC-mediated improvement in glycemic control in congenic animal models for obesity and obesity+T2DM/NIDDM and suggest that the CFC complex may be a useful nutraceutical adjunct in the dietary treatment of obesity and other glucose intolerant states as they occur in man and animals.
Keywords: obesity, diabetes, insulin resistance, fructan polymers, glycemic parameters, rats
Insulin sensitivity and carbohydrate metabolism. Diminished insulin sensitivity, also called insulin resistance (IR) occurs as one of several common sequala of obesity and Type 2 diabetes/non-insulin dependent diabetes (T2D, also NIDDM).1 The incidence and odds ratio linking obesity and T2DM/NIDDM in developed nations is now increasing to the point of becoming near alarming if not in epidemic proportions.2 Moreover, the economic cost of treating T2DM/NIDDM now consumes a major proportion of the health care expenditures in Western society and globally.2,3 In the USA alone, over one hundred million patients have been diagnosed with T2DM/NIDDM or prediabetic conditions as recently as 2014 with no end in sight to the rising incidence, at a cost of billions of dollars in direct costs to healthcare in addition to the lost productivity that becomes secondary to the disorder. The WHO has estimated that the prevalence of T2DM/NIDDM could reach 350million people worldwide by 2030 and impact a staggering further fiscal impact on the global health and wellbeing of the populations of all countries, where it is a significant contributing factor in the etiology of cardiovascular and kidney disease and their sequala.4 The application of animal models in obesity and T2DM/NIDDM research has shed considerable light on the various mechanisms which may be operative in the development of these disorders of Western civilization and include cellular and molecular aspects of the regulation of energy intake, energy expenditure, and insulin action at the receptor and post receptor levels of organization and function.5
Following ingestion of a carbohydrate meal, the digestive functions bring about denaturation and enzymatic hydrolysis of the carbohydrate polymers via α-glucosidase activity to yield monosaccharide glucose moieties, which readily undergo luminal absorption throughout the upper the small intestine of the gastrointestinal tract.6 The more complex the carbohydrate source ingested, the longer the absorptive process may take, although for most carbohydrate sources the ultimate luminal uptake is virtually 100% efficient and occurs primarily in the upper regions of the small intestine. For simple carbohydrates like the C-6 monosaccharide glucose, no further intestinal digestion or metabolism is necessary, and unlike other C-6 monosaccharides, the glucose moieties readily undergo virtually 100% luminal absorption unimpeded by other coexistent nutriments which may have been present in the meal.6,7 During a standard oral glucose tolerance (OGT) glucose is normally the only absorbable sugar present, and baring potential pathophysiologic factors, the absorbed glucose reaches the circulation in its entirety within 30minutes of ingestion where it can be easily measured. Once in the circulation, glucose moieties readily undergo uptake in peripheral tissues including muscle and adipose tissue, where insulin receptors become an essential intermediate in the internalization of the glucose moieties and where oxidation of the substrate can occur. The plasma concentrations of glucose after the 30-minute time point of an OGT thus represent the efficiency of peripheral uptake in muscle, adipose tissue, and other tissue depots, and are a valid indication of insulin sensitivity and insulin resistance in mediating glucose uptake in insulin sensitive tissues. Thus, the resulting plasma concentrations reflect the net glycemic index for the ingested nutriment, reflecting the magnitude of the glycemic load reaching the circulation and awaiting peripheral uptake and metabolic disposal. The greater the magnitude of the glycemic index and glucose area under the OGT curve, the greater the intensity of the insulin response that might be required to clear the circulatory loads of the glucose constituents of the meal. Chronically elevated circulating levels of insulin in concert with other hormonal actions contribute to decreased sensitivity to insulin actions including the efficiency of receptor binding of insulin in muscle and adipose tissue, the major insulin dependent tissues, with the result of a prolongation in plasma clearance of the glucose load imposed as occurs over time in the OGT test.1,6,7 In a normal OGT the pre-OGT glucose concentrations would be expected to return to at or near pre-OGT glucose concentrations well within 2hours of the glucose challenge. In states of insulin resistance however, the area under the OGT curve would be expected to be of greater magnitude than in animals exhibiting normal insulin sensitivity and glucose uptake in primary peripheral tissues including muscle and adipose tissue.
The complex saccharide inulin is a D-fructofuranosyl fructan (fructooligosaccharide) polymer with a single C-2 glucopyranosyl unit bonded to the terminal reducing end of the D-fructofuranosyl polymer thereby giving rise to a single digestible sucrose unit.7 Fructans have a comparatively low molecular weight (3,00-5,000 Da) compared to other starchy compounds and are fermented at least in part by intestinal microflora.8 Accordingly, inulin has a low glycemic index rating due to it containing the indigestible β-glycosidic polymer containing 20-30 fructose residues via the β-1-4 glycosidic linkages and with no known detrimental effects following human consumption. The lone terminal sucrose moiety generates equimolar quantities of glucose and fructose, where the fructose that is generated via initial α-glucosidase digestion may contribute to the perception of sweetness of the complex. Digestibility of carbohydrate polymers in mammalian organisms is typically limited to the C-2 linked D-glucopyranosyl unit positions on the terminal reducing end of the polymer and giving rise to an easily digestible terminal glucose or sucrose unit via a α-glucosidase activity. Limited additional degradation of the remaining fructan polymer and other oligofructan polymers may occur via action of intestinal microorganisms of the bifidobacterial, clostridia fusobacteria and other normal intestinal microbial species.9 Fructans are complex polymers containing fructose, a C-6 monosaccharide with a greater sweetness but a delayed luminal absorbability due to molecular differences at the C-2 carbon, which impede the normally efficient glucose absorption processes, resulting with fructose entering the enterocyte via facilitated diffusion, a slower process.6 Fructan polymers are commonly found in plants of the Compositae and Liliacae families, where they may be extracted from the roots, tubers, and bulbs of the plants.8 Additionally, fructans may also be isolated in smaller amounts from some species of algae and in numerous monocots and dicot plant sources including agave, asparagus, onions and garlic, leeks, barley, wheat, and others. The inclusion of inulin compounds into carbohydrate-rich and sweetened foods would be expected to attenuate the glycemic index of other nutriments which included the polymers and are referred to herein as the ‘CFC complex.’ In addition to the immediate effects of glycemic responses, because of their neutral physicochemical properties, fructans can remain in the gastrointestinal tract for some time, throughout much of their normal transit through the gut where they contribute to favorable effects on gastric emptying, viscosity of the luminal contents, and resulting in an attenuation in absorptive kinetics of other nutrients.9 Fructans are often combined with functional foods and nutritional supplements to contribute to an attenuation of the intestinal absorptive responses of meals and augment satiety reflexes. Chromium, sometimes referred to as ‘Glucose Transfer Factor (GTF) may enhance insulin mediated aspects of glucose uptake in insulin dependent tissues, where it has been proposed to enhance insulin-receptor interactions and in improved glucose uptake at the receptor level via interactions with the insulin receptor.10 In clinical trials addition of chromium to the treatment regimen was associated with improved glycemic control in NIDDM.11
The development of obesity occurs in numerous rodent animal models mostly as the result of an autosomal recessive trait, where it has been associated with impaired leptin actions in the CNS where defects in leptin binding and actions are proposed to result in hyperphagia in the obese phenotypes of the rodent strains12-14 These strains include those that bear the -cp trait originally derived from the -cp trait of the Koletsky rat by Hansen and characterized by Michaelis and others.12–15 Obesity becomes expressed in the LA/Ntul-cp and SHR/Ntul-cp strains by 5 to 6weeks of age and readily detected by alterations in gait, stance, physical appearance, and measurements of resting metabolic rates (RMRs) which are consistently lower than in their similarly reared lean littermates prior to significant differences in body weights of the two phenotypes.15 The -cp trait is expressed as an autosomal recessive epigenetic trait in the LA/Ntul//-cp and SHR/Ntul//-cp strains and occurs consistently in a multigenerationally fashion in 25% of the offspring of heterozygous breeders.12,13 The strains have been subjected to twelve cycles of backcrossing by Hansen to establish the congenic status of the strains and are believed to have been the first obese rat strains to have established the congenic status benchmark when the current colonies were established. The longevity of the lean phenotype of LA/Ntul//-cp rats has been observed to attain over 4years while the obese phenotype has been observed to live 3years.15 In contrast, the obese phenotype of the SHR/Ntul//-cp rats has rarely been observed to live beyond 15months of age. The obese of these strains develop numerous pathophysiologic characteristics known to be risk factors for obesity and T2DM/NIDDM linked disorders in one or both strains, including hyperamylinemia, hyperinsulinemia, hyperleptinemia, impaired nonshivering thermogenesis, hypertriglyceridemia, and in glomerulonephropathy with glycosuria and proteinuria, nerve conduction disorders, cataracts, impaired glycemic responses to a glucose challenge indicative of insulin resistance, and other pathophysiologic changes in the SHR/Ntul//-cp strain.16–18 The LA/Ntul//-cp strain remain non-diabetic throughout their known lifespan, while the SHR/Ntul//-cp strain develops T2DM/NIDDM by early adulthood or before.19 These congenic models represent unique models to investigate obesity-linked disorders in that the only known genetic difference between lean and obese littermates is the epigenetic expression of obesity beginning early in their postweaning development. The development of obesity in obese rodent has often been attributed to hyperphagia, but in a recent study hyperphagia in the lean littermate failed to produce obesity, suggesting that factors other than hyperphagia per se are a primary cause, and are reminiscent of the well-known Vermont Study of Obesity by Sims et al,19 where normally lean volunteers were overfed for long periods of time but who also failed to become obese by overfeeding alone. The specific nutritional components or other factors which contribute to the expression of the -cp trait and its sequala in the corpulent rat strains are little known.
The purpose of the current study was to determine if incorporation of a chromium-fructan polymer complex supplement with minimal digestibility (the ‘CFC complex’) could improve the OGT responses of normally reared SPF/VAF obese LA/Ntul//-cp and SHR/Ntul//-cp T2DM/NIDDM rats. This CFC complex is a common ingredient some nutritional supplements have sometimes suggested as a dietary supplement to control appetite, and which likely attributes its dietary actions to lower the magnitude of glycemic responses following ingestion of carbohydrate laden meals, especially those high in simple carbohydrates that add the appetite enhancing sensation of sweetness to the palate. The post-prandial glycemic responses of humans are likely to vary considerably, with or without the CFC complex included depending on the nutriment content of the meal itself, the level of obesity and any potential loss of insulin sensitivity in peripheral tissues that may be apparent, exercise levels, lifestyle factors, genetics including those that contribute to the ‘thrifty gene’ hypothesis, one’s chronologic age, and other aspects likely too numerous to quantify. Because of the genetic similarity in lean and obese members of these strains and the identical housing and rearing conditions of the animal subjects, these considerations help to confer an absence of non-obese and other extraneous traits that may be commonplace among human populations when interpreting the results of the study to the glycemic responses of administering an CFC complex during a controlled glucose challenge. Should the CFC complex be an effective nutraceutical at controlling post prandial glycemic responses in obesity, T2DM/NIDDM and other glucose intolerant conditions, its incorporation into meal planning and dietary practices could contribute to a reduction in the physical and financial burden to society resulting from these conditions.
Animal subjects and Housing. Male animals were selected from the Drexel colony of LA/Ntul//-cp and SHR/Ntul//-cp rats at 6weeks of age and separated into lean and pre-obese groups based on developing characteristics of the emerging obese or obese+T2DM/NIDDM state, which included early subtle changes in gait, stance, palpable fat and resting VO2. Selected animals were placed in shoebox cages lined with one inch of pine shavings, in a temperature (22-24 C) and humidity controlled (50-60% RH) environment from six until 12weeks of age. Animals were fed Purina Chow #4012 and house water ad libitum throughout the study. The Purina chow had a reported energy density of 3.34kcal/gram (14.2kjoules/gram) based on the manufacturer’s certificate of analysis and contained (w/w) 55.6% carbohydrate, 22.5% protein, 4.5% fat, 4.6% crude fiber, 6% ash, and 1-2 % essential vitamins and minerals and <5% moisture. Specific nutrient sources included ground extruded yellow corn, soybean meal, fish meal, cane molasses, wheat middlings, alfalfa meal, ground oats, brewer’s dry yeast, wheat germ meal, dried beet pulp, soybean oil, dicalcium phosphate , calcium carbonate, salt, and a blended vitamin supplement containing B-12 s, calcium pantothenate, choline chloride, riboflavin, thiamin, niacin, DL-methionine, D-activated animal sterol as a source of Vitamin D3, vitamin A, pyridoxine hydrochloride, vitamin E, calcium iodate, manganous oxide, ferrous carbonate, cobalt carbonate, copper sulfate, zinc sulfate, and zinc oxide. This diet has been deemed a complete life cycle diet especially formulated to support the growth, development, and maintenance of rats, including reproduction and lactation, and has been a standard rodent diet for biomedical research worldwide for many decades. The composition of the Chromium-Fructan complex (CFC) is depicted in Table 1 below.
Constituent |
g/100g_ |
Calcium (as CaCO3) |
8.06 |
Chromium* |
0.0065 |
Dahlia Root Complex |
32.261 |
Fructose |
40.3215 |
Glucose Polymers |
19.3510_ |
Total |
100.000_ |
Table 1 Chromium-Fructan Complex Composition
As chromium dinicotinate-glycinate and chromium picolinate, 1:1w/w mix, as originally incorporated in Dalulean™ wafers, obtained from (HPF, Trevose PA) and recommended as a dietary adjunct for appetite control.
Experimental Procedures: OGT and CFC preparation and administration. Animals were administered a 4-point oral glucose tolerance test (OGT) after a brief 4-hour period of food depravation, with free access to house water. The glucose challenge containing 2.50 grams glucose/kg of body weight as a 50% glucose solution was administered via intragastric gavage in the forenoon hours (1000-1200hr.) or subjected to the glucose challenge in the presence of the CFC complex (2.5g/kg body weight) or both glucose and CFC sequentially over a 1–5-minute duration. Bloods were obtained before (i.e., fasting) and 30, 60, 90, and 120minutes following the glucose or CFC challenge. Blood glucose concentrations were determined and recorded immediately after the blood draws with a rapid glucose oxidase method (Glucometer Elite®, Ames Laboratory, Elkhart IN). The glucose area under the OGT curve (AUC) was determined as described by Tulp et al as modelled after the procedure originally described by Wolever and used in our laboratory for many years.20,21 Plasma concentrations of Insulin and amylin were determined via radioimmunoassay using materials obtained from Penninsula Laboratories, Pomona CA.16 Hemoglobin A1C was determined spectrophotometrically following microcolumn resin separation of hemoglobin fractions using commercially prepared reagents and materials obtained from Sigma Chemical Company, St Louis, MO, USA. Data were analyzed via standard statistical procedures with the Stat view program, Abacus Concepts Berkley CA) and graphics accomplished with the Prism program (Prism Academy, San Diego, CA). The protocol and all experimental procedures were approved by the Institutional care and use committees prior to the conduct of this study.
Body weights and fasting glycemic parameters of rats. The body weights and some glycemic parameters of the animals studied are presented in Table 1 and indicate that the weights of obese animals were significantly greater than their lean littermates when 4 months of age, while the weights of the obese T2DM/NIDDM rats at 3months of age were modestly less than observed in the non-diabetic obese rats. Body weights of the old obese T2DN rats were significantly greater than all other groups studied, averaging twice the weights of the younger obese T2DM/NIDDM rats. Fasting glucose, insulin, and amylin concentrations were greater in the obese than in the lean LA/Ntul//-cp rats and were further increased in both young and old SHR/Ntul//-cp rats, consistent with their progressive development of diabetes. The percent hemoglobin A1c values were within normal concentrations in the lean and obese LA/Ntul//-cp rats are indicative of a long-standing normoglycemic status in both phenotypes despite their indication of insulin resistance as indicated by the greater Insulin to glucose ratio in the obese animals. This measure of insulin resistance indicated by the plasma insulin and glucose concentration was further deranged among the obese T2DM/NJDDM rats of both ages consistent with their diabetic state.
Glycemic parameters, including OGT and AUC in lean, obese and obese-T2DM/NIDDM rats. The OGT of lean and obese LA/Ntul//-cp rats are depicted in Figure 1 and indicate that the periodic glucose concentrations and the AUC of obese phenotype was greater than their lean littermates, consistent with moderate insulin resistance while remaining non-diabetic. The glucose concentrations and the AUC improved modestly when administered the CFC complex alone or in combination with the glucose challenge. In contrast, the OGT and AUC of the lean phenotype were similar with and without the CFC complex at all time points measured during the OGT. The OGT and AUC of obese T2DM/NIDDM rats of both ages were significantly greater than those of the non-diabetic obese rats and increased further in older obese T2DM/NIDDM rats. Addition of the CFC complex resulted in moderate decreases in the OGT and AUC in young diabetic rats, and significant decreases in both OGT and AUC glycemic parameters in the older T2DM/NIDDM rats (Figures 2 & 3). The CFC complex tended to extend the distribution in the presence of the glucose challenge, likely due to the incorporation of the more slowly luminal digestibility of glucose and fructan polymers. The glycemic responses to OGT were significantly more impaired in the obese T2DM/NIDDM rats of both ages than was observed in the non-diabetic obese animals. In addition, in female LA/Ntul//-cp rats offered sucrose supplement in their drinking water for up to 30months of age also failed to demonstrate characteristics of diabetes but did demonstrate an enhanced caloric efficiency throughout their lifespan, a marker of insulin resistance when contrasted to their obese littermates.18
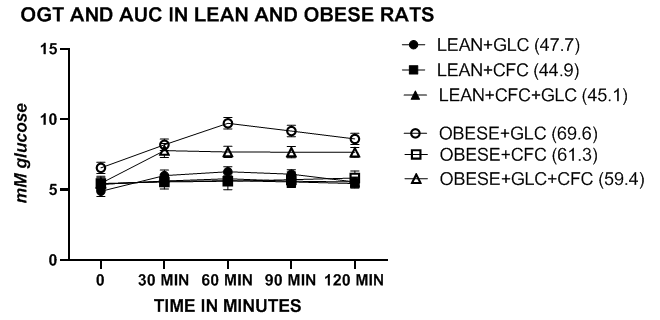
Figure 1 OGT and AUC of lean and obese LA/Ntul//-cp rats with glucose or CFC complex. Data are mean ±1 SEM, N= 6 animals/treatment group. The mean AUC of each treatment group is indicated in legend in parenthesis following each group.
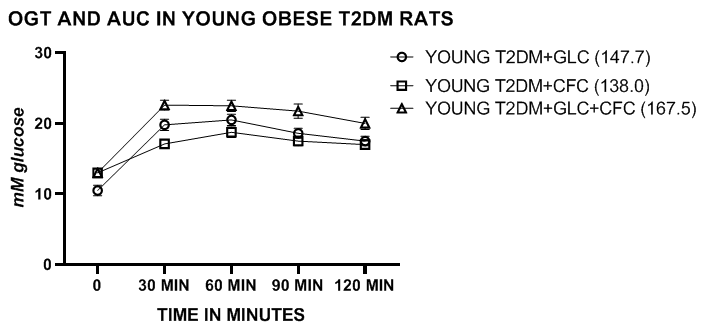
Figure 2 OGT and AUC of Young obese SHR/Ntul//-cp rats with glucose or CFC complex. Data are mean ±1 SEM, N= 6 animals/treatment group. The mean AUC of each treatment group is indicated in legend in parenthesis following each group. Note the different range of values on the vertical axis compared to Figure 1 above.
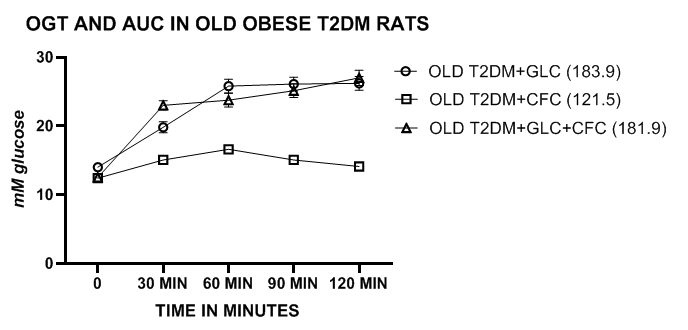
Figure 3 OGT and AUC of Old obese SHR/Ntul//-cp rats with glucose or CFC complex. Data are mean ±1 SEM, N= 5 animals/treatment group. The mean AUC of each treatment group is indicated in legend in parenthesis following each group. Note the different range of values on the vertical axis compared to Figure 1 above.
The prevalence of overweight and obesity are increasing in epidemic proportions in developed nations around the globe, along with the numerous pathophysiologic sequela that commonly accompany the excess adiposity and imposing a substantial human and fiscal burden on available resources not previously encountered.2-4 The basis for the excess adiposity is multifactorial in nature, as no single genetic, metabolic, or environmental cause beyond the obvious caloric imbalance has yet been identified as a primary cause. In addition, studies in human subjects often pose difficulties except for the likely exception of identical twins where genetic determinants are presumed virtually equivalent, and body weights and relative adiposity similar whether reared together or apart, thus attenuating in large part the possible differences experienced by variations in environmental or lifestyle factors.16 The environmental and genetic differences may be considerable among and between human populations, thus introducing potential variables not readily recognized and which may, depending on the experimental variables, be subject to introduction of unforeseen complications when attempting data analysis and interpretation. The congenic corpulent rat models present a unique opportunity to study individual factors however as the only obvious differences between the lean and obese phenotypes is the expression of the autosomal recessive -cp trait. The housing conditions and feeding regimens of all animals in the study were identical. The obese of both strains demonstrated measurable insulin resistance, a cardinal observation among obese animals and most obese human subjects and in the present study are attributable to the expression of the -cp trait, but only those animals in which the -cp trait was backcrossed into the SHR/N background developed obesity plus T2DM/NIDDM by early adulthood.22 The most prominent common factor between the two corpulent strains is the development of hyperinsulinemia at sometime early on during the postweaning growth stage and which in the present study was well established in the obese of both strains by 3-4months of age. The overall magnitude of insulin resistance in the obese differed between the two strains however and was significantly greater in the SHR/Ntul//-cp model, where it also continued to increase with age. In studies by Michaelis et al, long term administration of a high sucrose failed to induce diabetes in obese LA/N-cp rats by 10months of age, the end point of that investigation.22 As depicted in Figures 1-3 above, the glycemic responses to OGT were significantly more impaired in the obese T2DM/NIDDM rats of both ages than was observed in the non-diabetic obese animals. In addition, when female LA/Ntul//-cp rats offered sucrose supplement in their drinking water for up to 30months of age they also failed to demonstrate characteristics of diabetes but did demonstrate an enhanced caloric efficiency throughout their lifespan, a marker of insulin resistance when contrasted to their lean littermates.18 The differences in insulin sensitivity in the presence of elevated circulating insulin concentrations have been reported at least in part to a decreased efficiency of GLUT-4 transporter migration from the endoplasmic reticulum to the plasma membranes of muscle and adipose tissues and a partial oxidative shift from carbohydrates to non-carbohydrate substrates with corresponding proportionate increases in lipogenesis.23 The lipids can enter storage depots more energetically efficiently than glucose, as they do not require the GLUT-4 or other high energy linked transmembrane transport systems.24 In contrast, fructose utilizes insulin independent GLUT-5 receptors for intracellular uptake, where the fructose moieties can readily enter glycolysis.6
The increases in plasma insulin concentrations were also associated with increases in plasma amylin, an active peptide hormone that is typically co-secreted with insulin and plays a role in the regulation of gastric emptying and is thus linked to satiety responses.11 Obese animals of this and other obese rodent strains demonstrate hyperphagia, however, and the elevations in plasma amylin concentrations in the present study may also be an indication of amylin resistance. Amylin receptors are located in the antrum section of the stomach, and by slowing the rate of gastric emptying can help to normalize appetite, but in the presence of amylin insensitivity secondary to the elevated levels present in the obese animals, impede the satiety responses, and enable hyperphagia to occur. The fate of the excess calories consumed, independent of the macronutrient source becomes an easy target for fat accumulation among obese animals, and which excess fat accumulation may further impede carbohydrate linked insulin actions.
Phenotype |
n |
Weight, g |
Fasting Glucose |
Fasting Insulin |
Fasting Amylin |
HbA1c |
Lean |
6 |
365±18a |
4.97±0.16a |
0.62±0.10a |
5.2±0.08a |
2.8±0.2a |
Obese |
6 |
554±23c |
6.06±0.22b |
14.7±1.5b |
23.7±2.6b |
3.2±0.3a |
Young Obese-Diabetic |
6 |
295±10b |
12.50±1.60c |
39.3±3.0c |
50.5±5.5c |
N.D. |
Old Obese-Diabetic |
5 |
750±22d |
14.90±2.00c |
78.4±4.1d |
66.7±4.9d |
N.D. |
Table 2 Body weights and glycemic characteristics of lean, obese, and obese-diabetic rats
OGT and AUC 4month of age in lean and T2DM/NIDDM rats and old (12months) obese T2DM/NIDDM rats. Glucose reported in mM, Insulin as ng/ml, Amylin as pM, and HbA1c as %. The letter following each values indicate difference at p=<0.05 from other groups.
The absorptive phase of the OGT was delayed in obese of both strains, with the greatest extension in the obese-T2DM/NIDDM rats of both ages and only partially restored in the presence of the CFC complex. The OGT responses may be partially a reflection of the glucose and fructan polymers present in the CFC formulation. Glucose polymers in the form of polycose, with a molecular weight of approximately 5,000 Da, are commonly used in carbohydrate feeding formulations, where they can be utilized to be an easily but more slowly digestible caloric additive similar to partially hydrolyzed cornstarch without inducing undue luminal osmotic or digestive distress.9 The fructan polymers however impose a different digestive impact however as the bulk of fructan digestion occurs via microbial actions in the large intestine, and the monosaccharide moieties thereby generated but not directly oxidized by the colonic actions may enter the circulation much later in the digestive processive, and thus contribute little to the OGT glycemic responses, including the multiple insulin-dependent aspects of non-shivering thermogenesis and the capacity for energy expenditure, which have also been shown to be impaired in both obese strains of this and other studies.5,15,17,20,22 The contributions of the CFC complex on the absorptive phases of the glycemic responses to a glucose load resulted in improvements in the glycemic responses, resulting in lower peak glucose concentrations and a decrease AUC in obese animals, while in lean animals the decreases in glycemic responses and AUC were minimal, including the fasting insulin, amylin, and hemoglobin A1c concentrations. The HbA1c is formed via a non-enzymatic protein-glycosylation process and is reflective of the average ambient 24-hour glucose concentrations generated over the lifespan of the RBC, which is 3 to 4months duration in many mammalian species including humans. In other studies, the HbA1c concentrations of obese SHR/N-cp rats have been observed to remain elevated throughout much of the lifespan in that strain but were not quantified in the present study.17
The results of the present study indicate that inclusion of a chromium-fructan complex administered during a carbohydrate challenge can improve the glycemic parameters during an oral glucose tolerance test in two congenic obese and obese-diabetic rat strains that share the same origin of the epigenetic -cp trait for obesity. These observations further suggest that the CFC complex employed may be a useful nutraceutical adjunct in the dietary treatment of obesity and other glucose intolerant states as they occur in man and animals. The prevalence of obesity and its numerous pathophysiologic sequelae are now approaching epidemic proportions in many Westernized nations, where the consequences of obesity including T2DM/NIDDM, chronic renal and cardiovascular disease impose a heavy burden on the medical and financial resources of those nations. The dietary inclusion of complex carbohydrates and fiber remains one of the mainstays of a balanced diet, and which can contribute to normalization of glycemic parameters of meal ingestion, including improvements in insulin sensitivity and glucose uptake in muscle and adipose tissue, where most of the insulin resistance of obesity occurs. Inulin is an indigestible fructan polymer often found in numerous low glycemic index foods. As such, fructan complexes are often included in the composition of functional foods, where they contribute to a beneficial propagation and activity of the bifidobacterial microorganisms while suppressing the multiplication of the less healthful fusobacteria and facilitating maintenance of a healthy colonic pH via their fermentative actions. In addition, dietary fructans also contribute to the physicochemical properties, luminal viscosity, and organoleptic characteristics while attenuating the intestinal nutrient absorptive capacity of meals. Normalization of the glycemic response to meals is a desirable element in dietary planning in overweight, obesity and glucose intolerant states and implemented over time may contribute to a reduction of the metabolic and pathophysiologic sequela associated with obese, prediabetic and diabetic conditions. While the specific molecular or biochemical mechanisms of the improvements in post prandial glycemic cannot be determined from the present study, it is concluded that the inclusion of the CFC complex may prove to be a useful adjunct in the dietary management of impaired glycemic responses to ingested meals in man and animals.
The author acknowledges Ms. Mary Victor and Mr. Huang Peisong for animal care and husbandry, Dr Steve Dubin for veterinary care, and Ms. Susan Krasner for assistance in data collection, analysis, and data processing. The author acknowledges Professors Aftab Awan and George Einstein of USAT and UHHVI for editorial assistance in reviewing this manuscript and data analysis. In addition, the author wants to thank Drexel University, the University of Science Arts and Technology, Montserrat and the University of Health and Humanities, Virgin Islands for Institutional resources to complete these studies.
The authors declare no conflicts of interest related to this article.
Support by USAT Montserrat, UHHVI BVI, the Einstein Medical Institute and HPF, Trevose PA USA.

©2022 Tulp, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.