eISSN: 2575-906X


Research Article Volume 4 Issue 3
1Department of Animal Biology and Physiology, University of Yaounde I, Cameroon
2Department of biological sciences, University of Montreal, Canada
Correspondence: Billy Nguembock, Laboratory of Zoology, Ornithology Unit, Department of Animal Biology and Physiology, University of Yaounde I, P. O. Box 812 Yaounde, Cameroon, Tel +1-514-649-5791/+237-674-59-08-19
Received: April 15, 2020 | Published: June 17, 2020
Citation: Nguembock B, Guehoada Y, Mahamat S, et al. The birds of Cameroon: a bird survey from the Febe mountain (Centre region, Cameroon) coupled with a diversity analysis confirm a higher bird diversity in mountains compared to lowlands in this region of the Congo Basin Forest. Biodiversity Int J. 2020;4(3):140-149. DOI: 10.15406/bij.2020.04.00176
We conducted a bird survey in the Febe mountain in Cameroon in the Congo Basin. To obtain abundances for the birdlife, we used the mist-netting method and caught birds for seven months. In order to analyse the diversity and distribution of the avifauna, we carried out analyses aimed at obtaining indices of diversity and distribution as implemented in SAS/STAT and PAST software. We caught 187 individuals belonging to 19 families of birds and compared to previous surveys in this relictual mountain, we recorded two new genera, Iduna and Macrosphenus as well as three new species, Iduna pallida, Ploceuspelzelni and Macrosphenusconcolor; many of the birds captured were sedentary and the rest were seasonal, partial and intra-African migrants as well as migratory birds from the Western Palearctic. With regard to bird diversity, the values of the diversity indices show that the Febe mountain has a great diversity of birds; our results thus reveal an absence of supremacy of a species and suggest a large number of species within this mountain (H’=3.24).With the value of the Simpson diversity index of 0.94, our result indicates an abundance of the bird species within this birdlife certainly favoured by less competition between individuals due to the presence of the vegetation in the Febe mountain although it is a semi-deciduous forest degraded by human activities and the value of the Equitability index (J’=0.87) reveals an equal distribution of the individuals in this mountain. The Shannon-Wiener index as well as the Dominance of the three mountains investigated, including the Febe mountain, compared to those of the lowland investigated (Ekoko II village) confirm a higher bird diversity in the mountains particularly with a high vegetation cover while the Dominance of the lowland appeared in a preliminary manner more pronounced than that of the mountains but not with a real difference when the vegetation is strongly fragmented.
Keywords: bird survey, SAS/STAT and PAST software, diversity and distribution indices, diversity, dominance
Country of the Africa continent, Cameroon is located at the hinge of the Equatorial rainforest of Africa and according to WWF,1 Cameroon is one of the six countries that make up the Congo Basin Forest which is the second largest biodiversity reserve in the world after the Amazon. The Centre region of Cameroon is crossed by several mountains which are home to many animals and the first surveys in this region began during the colonization period.2 Located 250 kilometres from the coast of the Atlantic Ocean coast as well as from the northern edge of the large southern Equatorial Forest, the city of Yaounde is part of the South Cameroonian Plateau and is home to a big zoological park (Mvog-Betsi Zoo) in which an endemic fauna is preserved; this city is dominated by several relictual mountains including the Febe mountain (Figures 1&2).3–5

Figure 2 A view of the Febe Mountain taken from the Mbankolo district (Congo basin forest, Cameroon).
The Febe mountain is located in the second district of Yaounde and is bounded to the North by the district of Ntougou, to the South and West by the village of Febe and to the East by the district of Oliga. Mainly located in the Equatorial forest, this relict mountain has different types of vegetation such as the sub-montane forest, the secondary forest interspersed with scrubland and fallows due to human activities.6 As some mountains of this Centre region, the Febe mountain receives a lot of sun and precipitation and often constitutes, through its vegetation, a source of food for many birds. Several expeditions of naturalist ornithologists have thus taken place in this region and some specimens have been newly described.7–9 After general preliminary surveys in this Centre region, to date, no investigation concerning thebird survey on the relictual Febe mountain has only been deeply done for a relatively long time and published.10–14 However, it is important to note that so far in all bird surveys carried out in all Cameroonian regions, passerines are always more numerous than non-passerine birds.10–18
On the other hand, the distribution of species and their abundance depend on several types of factors. But according to MacArthur,19 environmental factors mainly affect the diversity of bird species and Telleria et al.,20 for example highlighted several factors that were involved in the distribution of forest species in the Iberian Peninsula. There is therefore a general link between the distribution of biodiversity and environmental factors. More specifically Thebault et al.,21 have shown that the relationship between the biodiversity and the ecosystem within a given environment is often linked to the food-web structure found in their habitats and Parsons et al.,22 similarlyestablished a clear correlation between feeding areas and the presence or absence of birds in an environment. Only since the bird surveys carried out in the Centre region of Cameroon, no study had clearly provided data on the correlation between the distribution of birds in this region and environmental factors before our studies.16–18
Our study, which was carried out in Mount Febe during seven months, has two main objectives: first, we deeply investigated the avifauna of this mount from base to summit and second, we tried to explore the diversity, abundance and distribution within the Febe avifauna to suggest hypotheses on the patterns of diversity and distribution in their living environment as well as we simply compared the diversity and distribution indices with those of other localities already obtained in this same region to make a preliminary comparative hypothesis on the diversity and Dominance of mountain and lowland avifauna.
Investigation of the Febe avifauna
To investigate the avi fauna, we used an appropriate method: the mist-netting method. This method is widely used for catching small to medium-sized wild birds such as passerines and shorebirds. According to this method, an inconspicuous mesh net is erected vertically on poles and deployed in areas of high activity to intercept birds as they go about their normal daily routines. In our captures, we used dark-coloured nylon nets and smaller mesh for smaller species. Otherwise, our mist nets have a series of three pockets running horizontally along the length of the net. Our mist nets were fixed with the mounting poles which had been chosen carefully and the choice of an appropriate mist-netting site was important for the capture success. Therefore, to ensure the capture success, we mainly identified their preferred flight paths, feeding areas, roosting and shaded sites. Generally, we start catching very early in the morning (5:00 AM) and we finish very late in the evening (sometimes 6:30 PM). To avoid a skew in our survey, we used the same eight mist nets in our different field missions and we did seven field missions during seven months.
Method for the calculation of the relative abundance of the Febe avifauna
To calculate the relative Abundance, we chose to use the Statistical Analysis System.23 We input dataas explained in the user guide and ran software until the obtaining of the results. Otherwise, we used Excel software program to obtain our histograms and curves.24
Method for the calculation of the occurrence of the Febe avifauna
In order to calculate the Occurrence, we used the same software, the Statistical Analysis System.23 As for the calculation of the relative Abundance, we input data as explained in the user guide and ran software until the obtaining of the results.
Methods of measuring the diversity and distribution of the Febe avifauna in their living environment and of comparing the diversity and the dominance of the mountain avifauna compared to that of lowland
Shannon-Wiener index (H’)
The Shannon-Wiener index represents the measure of the sum of degree of the uncertainty when it suggests predicting to which species would belong to an individual taken by chance in a collection of S species and N individuals. H’ = 0 if the community has only one species; H’ takes the maximal value log2S only when all species are represented by the same number of individuals. This index is determined by the following relationship:
pi= ni/N
Where pi = proportion of individuals of the species “i”; S = total number of species of the sample.
ni = number of individuals of the species “i”;
N = total number of individuals of the sample.
The Shannon-Wiener index (H’) increases when the number of the species of the community grows and, theoretically, it can reach elevated values. The value of H’ varies from 1 to log2S. In our study, the Shannon-Wienerindex was calculated with the PAST software.25
Simpson index (λ)
The Simpson index represents the proportion of abundance of the species “i”.25 This index measures the degree of concentration when individuals are classified into types. It is determined by the following relationship:
Where ni = number of individuals of the species “i”; n = total number of individuals of the sample.
Nevertheless, the most popular of these indices have been the inverse Simpson index (1/λ) and the Gini-Simpson index (1–λ) and both have also been called the Simpson index in the ecological literature. In our study, the Simpson index was calculated with the PAST software.25
Equitability index
The Equitability index measures the distribution of individuals within species independently to the specific richness. Its value varies from 0 (supremacy of one species) to 1 (equal distribution of individuals within species).
Thus, the Equitability index of Pielou (J’) is determined by the following formula:
J’ = H’/H’ max
H’ = Shannon-Wiener index
H’ max = log2S (S = the total number of species).
In our study, the Equitability index was calculated with the PAST software.25
All these indices have been obtained with a confidence threshold of 95%.
Method of comparing the diversity and the dominance of the mountain avifauna compared to that of lowland
After obtaining the Simpson index, we used it to calculate the Dominance (D = 1 – λ). D is a measure of dominance and it measures the extent of common species in the habitat; it ranges from 0 to 1. Furthermore, it is well known that the Shannon-Wiener index is a widely used index for comparing the diversity between various habitats.26 In the absence of significant sample, we proceeded to a simple comparison, by placing in a double entry table, on the one hand the values obtained in the only study carried out in the lowland and on the other, those obtained in the three different mountains already investigated in our laboratory and this in order to have a glimpse between the diversity and the Dominance of mountain avifauna compared to that of the low lands.
Abundance and occurrence of the Febe avifauna
Familial abundances of the Febe avifauna
In our sample, we captured187 individuals from 19 families of birds (Table 1). The most representative family is the Ploceidae family with 61 of 187 individuals (32.62%), followed by the Pycnonotidae family with 14.44% (Figure 3& Table 1).We found that 85.03% of the birdsbelonged to passerine families and 14.97% to non-passerine families (Table 1). The Estrildidae family was the more diverse with five genera and seven species, followed by the Pycnonotidae family represented by four genera and five species (Tables 2&3).
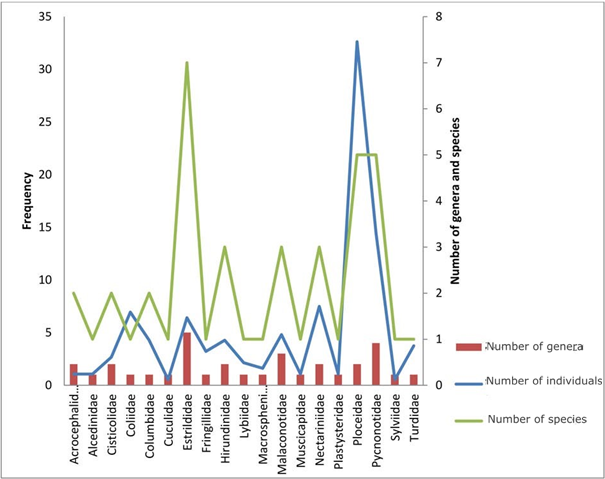
Figure 3 Relative familial abundance histogram in function of genera and species captured in the Febe Mountain of the Congo basin forest (Cameroon) during the bird survey between August 2015 and February 2016.
Families |
Absolute abundance |
Relative abundance (%) |
Acrocephalidae |
2 |
1,07 |
Alcedinidae |
2 |
1,07 |
Cisticolidae |
5 |
2,67 |
Coliidae |
13 |
6,95 |
Columbidae |
8 |
4,27 |
Cuculidae |
1 |
0,54 |
Estrildidae |
12 |
6,42 |
Fringillidae |
6 |
3,21 |
Hirundinidae |
8 |
4,27 |
Lybiidae |
4 |
2,14 |
Macrosphenidae |
3 |
1,61 |
Malaconotidae |
9 |
4,81 |
Muscicapidae |
2 |
1,07 |
Nectariniidae |
14 |
7,49 |
Platysteiridae |
2 |
1,07 |
Ploceidae |
61 |
32,62 |
Pycnonotidae |
27 |
14,44 |
Sylviidae |
1 |
0,54 |
Turdidae |
7 |
3,74 |
TOTAL |
187 |
100 |
Table 1 Familial abundances of the Febe avifauna obtained after the bird survey between August 2015 and February 2016 in the Febe mountain of the Congo Basin Forest (Cameroon)
Generic abundances and occurrences of the Febe avifauna
The187 individuals captured belong to 33 genera (Table 2). Within the Febe avifauna, themost representative genus was Ploceus (31.55%) (Ploceidae family). This genus is followed by Cinnyris(4.81%), Pycnonotus (4.81%), Chlorocichla (4.28%), Tchagra (3.74%), Turdus (3.74%), Atimastillas (3.74%), Crithagra (Ochrospiza) (3.21%), Psalidoprocne (2.67%), Cyanomitra (2.67%), Camaroptera (2.14%) and Nigrita (2.14%) belonging to the passerine families while genera Colius (6.95%), Turtur (4.28%) and Gymnobucco (2.14%) belong to non-passerine families (Figure 4 & Table 2). The underrepresented genera (relative abundance of 1%) belonging to passerine families are Acrocephalus, Chlorophoneus, Chrysococcyx, Cisticola, Cossypha, Euplectes, Iduna, Ispidina, Laniarius,Lonchura,Macrosphenus, Pyrenestes, Schisticolais and Spermophagawhile the others belonging to non-passerine families areIspidina and Chrysococcyx (Table 2).

Figure 4 Relative frequency histogram of genera in function of number of captured species in the Febe Mountain of the Congo Basin Forest (Cameroon) during the bird survey between August 2015 and February 2016.
Genera |
Absolute abundance |
Relative abundance (%) |
Frequency |
Occurrence (%) |
Acrocephalus |
1 |
0.53 |
1 |
14.29 |
Atimastillas |
7 |
3.74 |
5 |
71.43 |
Camaroptera |
4 |
2.14 |
2 |
28.57 |
Chlorocichla |
8 |
4.28 |
4 |
57.14 |
Chlorophoneus |
1 |
0.53 |
1 |
14.29 |
Chrysococcyx |
1 |
0.53 |
1 |
14.29 |
Cinnyris |
9 |
4.81 |
2 |
28.57 |
Cisticola |
1 |
0.53 |
1 |
14.29 |
Colius |
13 |
6.95 |
4 |
57.14 |
Cossypha |
2 |
1.07 |
1 |
14.29 |
Crithagra (Ochrospiza) |
6 |
3.21 |
3 |
42.86 |
Cyanomitra |
5 |
2.67 |
3 |
42.86 |
Estrilda |
4 |
2.14 |
4 |
57.14 |
Euplectes |
2 |
1.07 |
1 |
14.29 |
Eurillas |
3 |
1.6 |
1 |
14.29 |
Gymnobucco |
4 |
2.14 |
2 |
28.57 |
Iduna |
1 |
0.53 |
1 |
14.29 |
Ispidina |
2 |
1.07 |
2 |
28.57 |
Laniarius |
1 |
0.53 |
1 |
14.29 |
Lonchura |
2 |
1.07 |
2 |
28.57 |
Macrosphenus |
3 |
1.6 |
2 |
28.57 |
Nigrita |
4 |
2.14 |
2 |
28.57 |
Platysteira |
2 |
1.07 |
2 |
28.57 |
Ploceus |
59 |
31.55 |
7 |
100 |
Psalidoprocne |
5 |
2.67 |
3 |
42.86 |
Ptyonoprogne |
3 |
1.6 |
3 |
42.86 |
Pycnonotus |
9 |
4.81 |
5 |
71.43 |
Pyrenestes |
1 |
0.53 |
1 |
14.29 |
Schistolais |
1 |
0.53 |
1 |
14.29 |
Spermophaga |
1 |
0.53 |
1 |
14.29 |
Tchagra |
7 |
3.74 |
5 |
71.43 |
Turdus |
7 |
3.74 |
2 |
28.57 |
Turtur |
8 |
4.28 |
4 |
57.14 |
Totals |
187 |
100 |
/ |
// |
Table 2 Generic abundances and occurrences of all genera of the Febe avifauna captured during the bird survey between August 2015 and February 2016 in the Febemountain of the Congo Basin Forest (Cameroon)
36.36% of the genera sampled on theFebe mountain were rare (25% >FO≥10%) (Table 2). However within the most representative family Ploceidae, the genus Ploceus appeared very frequent on the Febemountain with a frequency of occurrence of 100% (Table 2). According to our results, three other genera were frequent in theFebe mountain among whichAtimastillas (FO=71.43%), Pycnonotus (FO=71.43%) andTchagra(FO=71.43%) (Table 2).
Specific abundances and occurrences of the Febe avifauna
According to our occurrence results, Ploceusnigerrimus appeared as the most frequent species with a frequency of occurrence of 100% (Table 3). In addition, 16.66% of the birds captured are frequently encountered on the Febe mountain, notably passerines Ploceuscucullatus (FO=85.71%), Atimastillasflavicollis (FO=71.43%), Ploceusnigricollis (FO=71.43%), Pycnonotus barbatus (FO=71.43%),Tchagraaustralis (FO=71.43%) and Chlorocichlafalkensteini (FO=57.14%) as well asa non-passerine member Coliusstriatus (FO=57.14)(Table 3). The least frequently encountered species(35.71%) included the members of passerines Cyanomitraverticalis (FO=42.86%), Estrildanonnula (FO=42.86%), Ptyonorprocnefilugula (FO=42.86%), Crithagramozambica (Ochrospizamozambica) (FO=42.86%), Camaropterabrachyura (FO=28.57%), Cinnyrisreichenowi (FO=28.57%), Macrosphenusconcolor (FO=28.57%), Nigritacanicapillus (FO=28.57%), Platysteiracyanea (FO=28.57%), Psalidoprocnepristoptera (FO=28.57%) and Turduspelios (FO=28.57%) as well as the non-passerine birds Ispidinapicta(FO=28.57%), Turturafer (FO=28.57%), Turturtympanistria (FO=28.57%) and Gymnobuccobonapartei (FO=28.57%) (Table 3). On the other hand, our analyses clearly show that several species (42.85%) sampled on theFebe mountain were rare (25% >FO≥10) (Table 3).
Scientific name |
Common name |
Absolute abundance |
Relative abundance (%) |
Frequency |
Occurrence (%) |
Acrocephalusbaeticatus |
African Reed-warbler |
1 |
0.53 |
1 |
14.29 |
Atimastillasflavicollis |
Yellow-throated Greenbul |
7 |
3.74 |
5 |
71.43 |
Camaropterabrachyura |
Green-backed Camaroptera |
4 |
2.14 |
2 |
28.57 |
Chlorocichlafalkeinsteini |
Yellow-necked Greenbul |
1 |
0.53 |
4 |
57.14 |
Chlorocichla simplex |
Simple Greenbul |
7 |
3.74 |
1 |
14.29 |
Chlorophoneusbocagei |
Bocage’sBushshrike |
1 |
0.53 |
1 |
14.29 |
Chrysococcyxcupreus |
African Emerald Cuckoo |
1 |
0.53 |
1 |
14.29 |
Cinnyrischloropygius |
Olive-bellied Sunbird |
1 |
0.53 |
1 |
14.29 |
Cinnyrisreichenowi |
Northern Double-collared Sunbird |
8 |
4.28 |
2 |
28.57 |
Cisticolaerythrops |
Red-faced Cisticola |
1 |
0.53 |
1 |
14.29 |
Coliusstriatus |
Speckled Mousebird |
13 |
6.95 |
4 |
57.14 |
Cossyphaniveicapilla |
Snowy-crowned Robin-chat |
2 |
1.07 |
1 |
14.29 |
Crithagramozambica |
Yellow-fronted Canary |
6 |
3.21 |
3 |
42.86 |
Cyanomitraverticalis |
Green-headed Sunbird |
5 |
2.67 |
3 |
42.86 |
Estrildaastrild |
Common waxbill |
1 |
0.53 |
1 |
14.29 |
Estrildanonnula |
Black-crowned Waxbill |
3 |
1.6 |
3 |
42.86 |
Euplectesardens |
Red-collared Widowbird |
2 |
1.07 |
1 |
14.29 |
Eurillasvirens |
Little Greenbul |
3 |
1.6 |
1 |
14.29 |
Gymnobuccobonapartei |
Grey-throated Barbet |
4 |
2.14 |
2 |
28.57 |
Iduna pallida |
Eastern Olivaceus Warbler |
1 |
0.53 |
1 |
14.29 |
Ispidinapicta |
African Pygmy-kingfisher |
2 |
1.07 |
2 |
28.57 |
Laniariusluehderi |
Luehder’sBushshrike |
1 |
0.53 |
1 |
14.29 |
Lonchura bicolor |
Black-and-white Mannikin |
1 |
0.53 |
1 |
14.29 |
Lonchura(Spermestes)cucullata |
Bronze Mannikin |
1 |
0.53 |
1 |
14.29 |
Macrosphenusconcolor |
Grey Longbill |
3 |
1.6 |
2 |
28.57 |
Nigritacanicapillus |
Grey-headed Negrofinch |
4 |
2.14 |
2 |
28.57 |
Platysteiracyanea |
Brown-throated Wattle-eye |
2 |
1.07 |
2 |
28.57 |
Ploceuscucullatus |
Village Weaver |
31 |
16.58 |
6 |
85.71 |
Ploceusnigerrimus |
Viellot’s Black Weaver |
11 |
5.88 |
7 |
100 |
Ploceusnigricollis |
Black-necked Weaver |
16 |
8.56 |
5 |
71.43 |
Ploceuspelzelni |
Slender-billed Weaver |
1 |
0.53 |
1 |
14.29 |
Psalidoprocnefuliginosa |
Mountain Saw-wing |
2 |
1.07 |
1 |
14.29 |
Psalidoprocnepristoptera |
Black Saw-wing |
3 |
1.6 |
2 |
28.57 |
Ptyonoprognefuligula |
Large Martin Rock |
3 |
1.6 |
3 |
42.86 |
Pycnonotus barbatus |
Common Bulbul |
9 |
4.81 |
5 |
71.43 |
Pyrenestesostrinus |
Black-bellied Seedcracker |
1 |
0.53 |
1 |
14.29 |
Schistolaisleucopogon |
White-chinned Prinia |
1 |
0.53 |
1 |
14.29 |
Spermophagahaematina |
Western Bluebill |
1 |
0.53 |
1 |
14.29 |
Tchagraaustralis |
Brown-crowned Tchagra |
7 |
3.74 |
5 |
71.43 |
Turduspelios |
African Thrush |
7 |
3.74 |
2 |
28.57 |
Turturafer |
Blue-spotted Wood Dove |
3 |
1.6 |
2 |
28.57 |
Turturtympanistria |
Tambourine Dove |
5 |
2.67 |
2 |
28.57 |
Totals |
|
187 |
100 |
/ |
// |
Table 3 Specific abundance and occurrence of each captured species of the Febe avifauna during the bird survey between August 2015 and February 2016 in the Febemountain of the Congo Basin Forest (Cameroon)
Diversity indices
The Shannon-Wiener index was 2.34 at the family level, 2.80 at the generic level and 3.24 at the specific level (Table 4). As for the Simpson index, the values were 0.84 at the family level, 0.87 at the generic level and of 0.94 at the specific level (Table 4). As for the Shannon-Wiener index, the general trend is the same as the Simpson index with the values obtained which are also high (Table 4). The Equitability index values were 0.79 at the family level, 0.80 at the generic level and 0.86 at the specific level (Table 4). As forother indices, the values obtained also appear far from 0 (Table 4).
Taxonomic level |
Family |
Generic |
Specific |
Taxa S |
19 |
33 |
42 |
Individuals |
187 |
187 |
187 |
Simpson (λ) |
0.84 |
0.87 |
0.94 |
Dominance (D) |
0.16 |
0.13 |
0.06 |
Shannon-Weiner (H') |
2.34 |
2.8 |
3.24 |
H'max |
2.96 |
3.5 |
3.76 |
Equitability (J) |
0.79 |
0.8 |
0.86 |
Table 4 Diversity indices of the Febe avifauna within their life environment obtained from the PAST software25
Comparison of the diversity and the dominance of the mountain avifauna to that of lowland
By putting side by side the values of diversity and Dominance of mountain avifauna on the one hand and those of the lowland on the other, we found that the avifauna ofthe Febe mountain is slightly more diverse(H’ = 3.24) than that ofthe Ekoko II village (H’ = 3.14) and at the same time we noted that their Dominance are almost identical respectively (D = 0.06) and (D = 0.07) even if that of the Ekoko II village remains higher (Table 5).
In the second case, the avifauna ofAbobo-Etetak hill is more diverse (H’ = 3.34) than that ofthe Ekoko II village (H’ = 3.14) and at the same timewe noted that the Dominance of theEkoko II village is more pronounced (D = 0.07) than that of Abobo-Etetak hill (D = 0.05) (Table 5).
|
|
Mountains |
|
|
|
|
|
|
|
Abobo-Etetak Hill |
EloumdenMountain |
FebeMountain |
|||
Shannon-Weiner diversity index (H’) |
Dominance (D) |
Shannon-Weiner diversity index (H’) |
Dominance (D) |
Shannon-Weiner diversity index (H’) |
Dominance (D) |
||
Lowlands |
3.34 |
0.05 |
3.62 |
0.04 |
3.24 |
0.06 |
|
|
Ekoko II village |
3.14 |
0.07 |
3.14 |
0.07 |
3.14 |
0.07 |
Table 5 Values used to compare the diversity and Dominance of the mountain avifauna versus that of the lowland
Legends: Blue color for the diversity of mountain avifauna; Green color for the Dominance of lowland avifauna.
In the third case, the avifauna of the Eloumden mountain is much more diverse (H’ = 3.62) than that of the Ekoko II village (H’ = 3.14) and at the same time we noted that the Dominance of the Ekoko II village appears much more pronounced (D = 0.07) than that of the Eloumden mountain (D = 0.04) (Table 5).
Avifauna of the Febemountain
As with all surveys carried out so farin the Centre region of Cameroon,11–18 the survey on the Febe mountain confirms the same distribution of birds as those already carried out in this same region with passerines (85.03%) more numerous than non-passerines (14.97%) (Table 1 & Figure 3). Some families of non-passerinessuch as Coliidae, Columbidae, Cuculidae and Lybiidae have already beenindicated and several families of passerines among whichEstrildidae, Fringillidae, Ploceidae, Pycnonotidae, Sylviidae andTurdidae have also been mentioned in this region.11–18 But until now, several representatives of some families of passerines, highlighted by our study, were not yet indicated as present in the relictual Febemountain.12,13
In thispresent study, we havehighlighted, more generally in this Centre region and in particular in the Febe mountain, new taxonomic representatives of several families of passerines; compared to all previous surveys conducted in this region,11–18 more specifically, we have newly highlighted genera andspecies belonging tothree families of passerines, Acrocephalidae, Macrosphenidae and Ploceidae (Table 1) (see details below).
According to our results (Tables 2&3), two new genera, Iduna and Macrosphenus, as well as three new species (details below), so far not recorded in the Centre region of Cameroon and therefore in the relictualFebe mountain, were identified during our investigation. It is: Iduna pallid Hemprich & Ehrenberg, 1833 belongs to the family Acrocephalidae27 (Figure 5): the eastern olivaceous warbler is a small passerine certainly originating in the Old World but now distributed in Europe, in the Middle East, Asia and Africa. In Cameroon, this small passerine was, until this study, only found in the Far North region.28 This medium-sized warbler is a migratory bird that winters in sub-Saharan Africa or Arabia and its song is a rapid nasal babbling.29
Macrosphenusconcolor Hartlaub, 1857 belongs to the family Macrosphenidae27 (Figure 6): this African warbler has a very widedistribution and is variously distributed. In Cameroon, the grey longbill is largely found inthe Centre and South regions while it is absent in the North regions.28 According to del Hoyo et al.,29 this species is a sedentary bird and its diet is mainly composed of insects, including moths and small caterpillars as well as small grasshoppers.
Ploceuspelzelni Hartlaub, 1887 belongs to the family Ploceidae27 (Figure 7): endemic to Africa, the slender-billed weaver is a species of bird found only in Africa central and western. In Cameroon, this weaver is found variously in some regions such as the Littoral, the North-West, etc. and not in others like the East, the Far North, etc. but especially in the Centre region investigated.28 This very small weaver (11 cm; 10 to 16 g) is an African resident.28,29 Otherwise, we noted, in this species, swizzling calls (Billy Nguembock personal observation).
Distribution and diversity of the avifauna in the Febemountain
Based on our results, we note an absence of supremacy of a species along the Febe mountain and species appear practically with the same distribution (J’= 0.87 and H’ = 3.24; Table 4). According to some authors, the distribution pattern of speciesin their living environment may depend on historical factors in this case their evolutionary adaptation30,31 but for others, rather in function of the ecological and biological factors.32–35 The Febe mountain, althoughlocated in the Equatorial rainforest, shows a vegetation in places with a fragmented foreststrongly degraded by human activities (Figure 2); this mountain receives a lot of precipitationalmost all the year andtherefore offersfavorable conditionswith permanentlya green landscapedottedwith semi-deciduous forest mixed,in certain places, with plantations from the base to the top. Thus, the distribution patternof the birdlife in the Febemountain would be linked to ecological factors in this case the vegetation. According to Sagar in and Gaines,36 precipitation has an impact on bird habitats by generating food and the availability of cover through vegetation; this could explain why we found in the Febe birdlife a mixture of specialist (poor dispersers), generalist (better dispersers) and even migratory birds. This hypothesis is corroborated by several studies carried out in some regions of Cameroon in whichauthorsfound that the distribution ofthe birdlifeis directly linked tovegetation maintained by precipitation that falls almost all year round and constantlymaking food available to birds.13–18 And beyond Cameroon, Azman et al.,37 in Malaysia found that bird diversity was higher in areas with high vegetation diversity such as primary and secondary forests. Thus, with the Febe mountain which shows a vegetation variously composedfrom the base to the summit, the Simpson index (λ = 0.94; Table 4) supports that species were abundantly represented in the Febe birdlife with an equal distribution of the individuals inside species (J’= 0.87;Table 4). Furthermore, the Shannon-Wiener index with an obtained value of H’ = 3.24, which moves away from 1, expresses the high number of the species within the Febe mountain and therefore suggests that the bird diversity was high in this relictual mountain. All these indices ofdiversity tend to confirm our first resultsconcerning the great diversity of birdsin this Centre region of Cameroon, region ofthe Congo Basin which is the second greatest reserve in terms of biodiversity in the world.16–18
Comparison of the diversity and the dominance of the mountain avifauna to that of lowland
From our results on the comparison of the diversity and Dominance of mountain and lowland birdlife, we found thatthe diversity was higher inmountains first in the Eloumden mountain, then on theAbobo-Etetak hill and finally inthe Febe mountain whileit wasnotably lower for the Ekoko II lowlandandconversely, Dominance was more pronounced in theEkoko II lowland, followed respectively by the mountains Febe, Abobo-Etetak (hill) and Eloumden (Table 5).
According to several authors,38–40 the diversity of bird species is higher in areas where the vegetation cover is also higher. Among the three mountains investigated, we noted that the vegetation of the Eloumden mountain is a forest certainly degraded by human action but often covered by the presence of trees characteristic of the primary forest while for the other two, the vegetation of the Abobo-Etetak hill is a secondary forest well degraded by human activities, in particular agriculture although many pockets of forest are still observed and that of the Febe mountain is mainly a semi-deciduous sub-montane forest showing strong anthropogenic disturbances.4,6,41,42 Thus, our results on the diversity of the avifauna of the different sites investigated strongly corroborate the conclusion suggested by several authors such as38,39,40 (Table 5). For the Ekoko II lowland characterized by the absence of realvegetation cover, the diversity of bird species was the lowest of all the sites investigated in this region confirming our first results.18
Dominance implies that few species predominate in habitats and this can be determined by a combination of factors, including an absence or reduction of vegetation and therefore a food unavailability.40,43 To concretely analyse the predominance of the species of each site beyond the value already obtained, we chose to add the relative abundances of the three most represented species of each site studied and these results follow the same trend as those presented (Table 5). The results revealed that for the Ekoko II lowland, the first three species belong to the same family and their total gave the highest percentage (39.64%) for Ploceusnigerrimus, P. nigricollis and P. cucullatus;18 this family appeared predominant on this site expressing a less diversified vegetation cover and therefore offering a low range of food. The Febe mountainranked behind the lowland Ekoko II with a lower percentage (32.09%) for the species Ploceuscucullatus, P.nigricollis and Coliusstriatus (Table 3) while the Abobo-Etetak hill ranked behind the Febe mountain with a slightly lower percentage (30.88%) for Ploceuscucullatus, Euplectesardens and Quelea erythrops;16 in these two sites, we noted the predominance of Ploceuscucullatus which feeds mainly seeds, seeds of grasses, grains, cultivated cereals, etc. illustrating a slightly diversified vegetation with the plantations from where besides a great representation of the frugivore Coliusstriatus in the two sites.16 The Eloumden mountain had the lowest percentage (20.25%) with the species Gymnobuccobonapartei, Pycnonotus barbatus and Ploceuscucullatus; these representatives of three different families with a mixture of specialist, generalist and migratory birds testify to the great diversity of the vegetation cover offering a very wide range of food for birds in this mountain.17 Our results strongly corroborate those of certain authors such as40 and in particular of Marsden et al.,44 who recorded the predominance of some bird species in an open area in Brazil, which was the result of intensive clearing of the understorey vegetation resulting in unavailability of food.
This present study, which was carried out in a montanesite at a very low altitude (an altitude below 800 to 900 metres) confirms the same representativeness already obtained in the Centre region with passerines more numerous than non-passerines. Compared to previous surveys carried out in this same region of the Congo Basin Forest, our studyrevealedtwo new genera, Iduna and Macrosphenus, and three new species, Iduna pallida, Macrosphenusconcolor and Ploceuspelzelninow present in this region.Moreover despite a semi-deciduous sub-montane forest strongly altered by human activities, the distribution pattern within the Febe mountain remains linked to ecological factors,in this casethe vegetation.Finally, our current study on the comparison of the diversity and Dominance of mountain and lowland avifauna revealed that the diversity of bird species is higher in the mountains,in particular where the vegetation is more diversified and Dominance is more pronounced in an environment where the vegetation is less diversifiedorpossibly when the vegetation is heavily fragmented often due to anthropogenic disturbances.
We thank Mr.Essomba who guided and advised us during the sampling of birds in the field. We would also like to thank two anonymous referees for their comments on an earlier version of this manuscript. We will not forget to thank Mr.Wandji Alain Christel who helped set up the map for our study site.We also thank all theentire team of the Ornithology Unit within the Zoology Laboratory of the University of Yaoundé I in Cameroon for their contribution and their preciousadvice in therealization ofthis project.
Authors declare that there is no conflict of interest.

©2020 Nguembock, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.