International Journal of
eISSN: 2574-9862


Research Article Volume 4 Issue 4
1Department of Animal Biology and Physiology, University of Yaounde I, Cameroon
2Department of biological sciences, University of Montreal, Canada
Correspondence: Billy Nguembock, Laboratory of Zoology, Department of Animal Biology and Physiology, Ornithology Unit, University of Yaounde I, P. O. Box 812 Yaounde, Cameroon, Tel +00237694144294
Received: September 30, 2019 | Published: November 29, 2019
Citation: Nguembock B, Azang EDO, Mahamat S. Bird survey in a forest mountain of Congo Basin (Eloumden, Cameroon) and diversity analysis confirm high diversity of world second reserve and correlation between species diversity and vegetation. Int J Avian & Wildlife Biol. 2019;4(4):118-127. DOI: 10.15406/ijawb.2019.04.00163
Our knowledge of the Congo Basin Forest, which is composed of six countries including Cameroon, and which is the world second Reserve in terms of Biodiversity after the Amazon, is almost non-existent because of few studies carried out in this great region. Some shy studies focused on biodiversity surveys have been undertaken within a few countries for some groups such as Arthropods, Plants, Mammals, etc. but very little about Birds. Here we present data on the bird survey conducted in a region of Cameroon. To perform the bird survey, we used mist-netting method during six months to obtain the abundance of bird species. In order to analyse diversity and distribution, we accomplished analyses with SAS/STAT and PAST softwares. We caught 158 individuals belonging to 20 birds’ families, 70% of which came from passerine families and 30% from non-passerine. Compared with previous surveys in the same region, we newly recorded one genus, Bradornis, and seven species, Trachyphonus purpuratus, Psalidoprocne fuliginosa, Ptyonoprogne fuligula, Bradornis fuliginosus, Cinnyris batesi, Chalcomitra adelberti and Acrocephalus baeticatus. This high avifaunistic diversity is supported by estimated values of diversity indexes which show an absence of supremacy of one species (H’=3.62) and an equal distribution of individuals in their environment (J’=0.92). With the value of the Simpson diversity index of 0.96, our result confirms high diversity within the Eloumden avifauna. Our study confirms that the composition as well as the structure of the Eloumden mountain as observed in the field play a main role in the diversity of bird species at least at the local level. Based on our distribution and diversity analyses, the pattern of variation in distribution of birds in the Eloumden mountain appears to lean on environmental factors and particularly the vegetation, which played the main role with a bird mixture of poor and better dispersers, migratory and vagrant due to presence of luxuriant vegetation all year round, and this also explains the lesser competition noted (D = 0.96).
Keywords: congo basin forest, bird survey, mist netting method, distribution and diversity analyses, avifauna
The Congo Basin Forest is the world’s second-largest dense humid tropical forest after the Amazon.1 It extends from the coast of the Atlantic Ocean in the west to the mountains of the Albertine Rift in the east. It covers an area of approximately 2 millions square kilometres and spans across six countries among which is Cameroon. Because of its latitudinal extending, from the Equatorial rainforest in the heart of the Congo Basin Forest until Sahelian regions of the Lake Chad border, Cameroon is among the richest countries in Africa in terms of biodiversity particularly its diversified avifauna with over 970 species.2–10 This biological diversity is increased by the presence of several mountains around the country particularly those of the volcanic mountains’ chain in the Western Region but also the lowland of the Centre region.5,11 The Centre region shows the particularity of having a mixture of vegetation between the Equatorial forest and the montane district at very low altitude but it also counts several mountains as Eloumden mountain in which we investigated the avifauna. The Eloumden mountain is located in the North-West of Yaounde (Figure 1); this mountain stands at 1159 metres and is subdivided into two separate parts: Eloumden I and Eloumden II. Situated in Mbadoumou village, it is located in the Equatorial forest area in the Congo Basin; it has a great particularity of showing two types of hillsides: the hillside below the wind and the one in front of the wind.12 The hillside in front of the wind receives lots of sun and precipitations than the one below the wind and constitutes an abundant food source for many birds through its important vegetation.12 In addition, in this vegetation, we can note the presence of several types of seeds, various types of fruits including berries, many trees with flowers and other cultivated plants.
First surveys in this region began during the colonization period and some colonialists liked to organize gun parts.13 Several expeditions by naturalist ornithologists have thus taken place in this region of the Congo Basin and some specimens have even been newly proposed.14–16 After these preliminaries surveys, very few scientific expeditions have been organized in this region11,17 and just some birds’ surveys have been carried out2–4,18,19 although no wildlife survey has been particularly focused on the Eloumden mountain during a long period.4 Based on these few bird surveys, passerine representatives are systematically more numerous than non-passerine.3–5,18,19 As known now, relationships between the biodiversity and environment function through trophic interactions. Only it is more and more established that the relationship between biodiversity and an ecosystem within the environment is often related to the food-web structure found in these habitats.20 In the same way, Parsons et al.,21 have also established a correlation between food areas and the presence or the absence of birds and according to Abrams22 in a given environment the rise in productivity plays a great role on the abundances of the trophic levels and it has an impact on the biodiversity which is often increased. But the bird surveys carried out in different regions of Cameroon did not bring information on relationships between birds and their life environment particularly on the principle of taxa distribution in their environment as well as conditions of their abundance.2–5,19 In this study carried out in one of the six countries covering the Congo Basin Forest, we mainly followed two purposes: firstly, we thoroughly investigated the avifauna of this mountain which shelters a big Forest from base to tip and secondly, we tried to explore the diversity, abundance and distribution within the Eloumden avifauna to propose hypotheses on their patterns of distribution as well as reasons of their diversity or abundance if it is established in their biotic environment.
Investigation of the Eloumden avifauna
To investigate the avifauna, we used an appropriate method: the mist-netting method. This method is widely used for catching small to medium-sized wild birds such as passerines and shorebirds. According to this method, an inconspicuous mesh net is erected vertically on poles and deployed in areas of high activity to intercept birds as they go about their normal daily routines. In our captures, we used dark-coloured nylon nets and smaller mesh for smaller species. Otherwise, our mist nets have a series of 3 pockets running horizontally along the length of the net. Our mist nets were fixed with the mounting poles which had been chosen carefully and the choice of an appropriate mist-netting site was important for the capture success. Thus in order to ensure the capture success, we mainly identified their preferred flight paths, feeding areas, roosting and shaded sites. Generally, we start catching very early in the morning (5:00AM) and we finish very late in the evening (sometimes 6:30PM). In order to avoid a skew in our survey, we used the same eight mist nets in our different field mission and we did eight field missions during six months.
Method for the calculation of the relative abundance of the Eloumden avifauna
In order to calculate the relative abundance, we chose to use the Statistical Analysis System.23 We input data as explained in the user guide and ran software until the obtaining of the results. Otherwise, we used Excel software program to obtain our histograms and curves.24
Method for the calculation of the occurrence of the Eloumden avifauna
In order to calculate the occurrence, we used the same software, the Statistical Analysis System.23 As for the calculation of the relative abundance, we input data as explained in the user guide and ran software until the obtaining of the results.
Methods for the measure of the distribution of the Eloumden avifauna in line with their environment
Shannon index (H’)
The Shannon’s diversity index represents the measure of the sum of degree of the uncertainty when it suggests predicting to which species would belong to an individual taken by chance in a collection of S species and N individuals. H’ = 0 if the community has only one species; H’ takes the maximal value log2S only when all species are represented by the same number of individuals. This index is determined by the following relationship:
pi = ni/N
Where pi = proportion of individuals of the species “i”; S = total number of species of the sample.
ni = number of individuals of the species “i”;
N = total number of individuals of the sample.
The Shannon index (H’) increases when the number of the species of the community grows and, theoretically, it can reach elevated values. The value of H’ varies from 1 to log2S. In our study, the Shannon index was calculated with the PAST software.25
Simpson index (λ)
The Simpson index represents the proportion of abundance of the species “i”.25 This index measures the degree of concentration when individuals are classified into types. It is determined by the following relationship:
where ni = number of individuals of the species “i”; n = total number of individuals of the sample.
Nevertheless, the most popular of such indexes have been the inverse Simpson index (1/λ) and the Gini-Simpson index (1–λ) and both have also been called the Simpson index in the ecological literature. In our study, the Simpson index was calculated with the PAST software.25
Equitability index
The Equitability index measures the distribution of individuals within species independently to the specific richness. Its value varies from 0 (supremacy of one species) to 1 (equal distribution of individuals within species).
Thus, the Equitability index of Pielou (J’) is determined by the following formula:
J’ = H’/H’ max
H’ = Shannon index
H’ max = log2S (S = the total number of species).
In our study, the Equitability index was calculated with the PAST software.25
All these indexes have been obtained with a confidence threshold of 95%.
Abundance and occurrence of the eloumden avifauna
Familial abundances of the Eloumden avifauna
We caught 158 individuals belonging to 20 birds families (Table 1). The most representative family is the Pycnonotidae family with 31 of the 158 captured individuals (19.62%) (Figure 2 & Table 1). We found that 70% of the captures were from the passerine families and 30% from non-passerine (Table 1). The Pycnonotidae family was the more diversified with five genera and seven species, followed by the Nectariniidae family with four genera and eight species and the Estrildidae family with four genera and four species (Tables 2&3).
Families |
Absolute abundance |
Relative abundance(%) |
Alcedinidae |
3 |
1.9 |
Coliidae |
3 |
1.9 |
Columbidae |
9 |
5.7 |
Cuculidae |
2 |
1.27 |
Estrildidae |
9 |
5.7 |
Fringillidae |
1 |
0.63 |
Hirundinidae |
14 |
8.86 |
Lybiidae |
20 |
12.66 |
Malaconotidae |
7 |
4.43 |
Monarchidae |
4 |
2.53 |
Muscicapidae |
3 |
1.9 |
Nectariniidae |
20 |
12.66 |
Nicatoridae |
5 |
3.16 |
Picidae |
1 |
0.63 |
Platysteiridae |
1 |
0.63 |
Ploceidae |
15 |
9.49 |
Pycnonotidae |
31 |
19.62 |
Sylviidae |
5 |
3.16 |
Turdidae |
5 |
3.16 |
TOTAL |
158 |
100 |
Table 1 Familial abundance of the Eloumden avifauna obtained after the bird survey between September 2015 and February 2016 in the relictual Eloumden mountain of the Congo Basin Forest
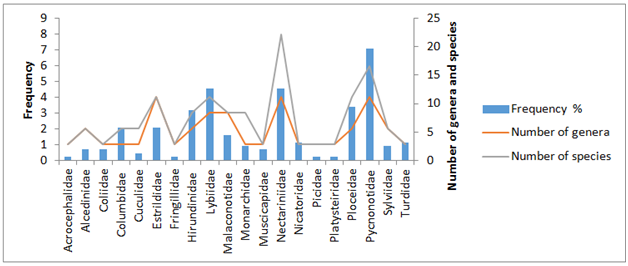
Figure 2 Relative familial abundance histogram in function of genera and species captured during the bird survey between September 2015 and February 2016 in the relictual Eloumden mountain of the Congo Basin Forest.
Generic abundances and occurrences of the Eloumden avifauna
The 158 captured individuals belong to 37 genera (Table 2). The most representative genera are those of two passerine, Eurillas (Pycnonotidae) and Ploceus (Ploceidae), and one non-passerine, Gymnobucco (Lybiidae) (Figure 3 & Table 2). The less representative genera with a relative abundance of 0.63% are those belonging to eleven passerine, Acrocephalus, Chalcomitra, Estrilda, Hedydipna, Laniarius, Lonchura, Mandingoa, Nicator, Ochrospiza, Platysteira and Thescelocichla, and the two remaining belong to non-passerine, Campethera and Halcyon (Table 2).
Genera |
Absolute abundance |
Relative abundance (%) |
Acrocephalus |
1 |
0.63 |
Amblyospiza |
2 |
1.27 |
Camaroptera |
2 |
1.27 |
Campethera |
1 |
0.63 |
Ceyx |
2 |
1.27 |
Chalcomitra |
1 |
0.63 |
Chlorocichla |
10 |
6.33 |
Chrysococcyx |
2 |
1.27 |
Cinnyris |
10 |
6.33 |
Colius |
3 |
1.9 |
Cyanomitra |
9 |
5.7 |
Dryoscopus |
3 |
1.9 |
Estrilda |
1 |
0.63 |
Eurillas |
13 |
8.23 |
Gymnobucco |
13 |
8.23 |
Halcyon |
1 |
0.63 |
Hedydipna |
1 |
0.63 |
Ptynoprogne |
2 |
1.27 |
Hylia |
2 |
1.27 |
Laniarus |
1 |
0.63 |
Lonchura |
1 |
0.63 |
Mandingoa |
1 |
0.63 |
Bradornis |
3 |
1.9 |
Nicator |
1 |
0.63 |
Ochrospiza |
1 |
0.63 |
Platysteira |
1 |
0.63 |
Ploceus |
13 |
8.23 |
Pogoniulus |
4 |
2.53 |
Psalidoprocne |
11 |
6.96 |
Pycnonotus |
11 |
6.96 |
Spermophaga |
6 |
3.8 |
Tchagra |
3 |
1.9 |
Terpsiphone |
4 |
2.53 |
Thescelocichla |
1 |
0.63 |
Trachylaemus |
3 |
1.9 |
Turdus |
5 |
3.16 |
Turtur |
9 |
5.7 |
TOTAL |
158 |
100 |
Table 2 Generic abundance of the Eloumden avifauna obtained after the bird survey between September 2015 and February 2016 in the relictual Eloumden mountain of the Congo Basin Forest

Figure 3 Relative frequency histogram of genera in function of number of captured species during the bird survey between September 2015 and February 2016 in the relictual Eloumden mountain of the Congo Basin Forest.
According to Dajoz26 43.24% of the genera sampled were rare. However within the most representative Pycnonotidae family, genera Eurillas and Chlorocichla appeared very frequent, both of them having an occurrence of 75%. According to our results, four other have been frequent; it was about Ploceus, Cinnyris, Cyanomitra and Gymnobucco.
Specific abundances and occurrences of the Eloumden avifauna
According to our occurrence results, only 13.72% of captured birds are relatively common including five passerine members, Chlorocichla simplex, Eurillas latirostris, Pycnonotus barbatus, Cinnyris superbus and Spermophaga haematina, and one non-passerine member, Gymnobucco bonapartei (Table 3). According to Dajoz26 species less commonly encountered included fourteen passerine members, Ambyospiza albifrons, Camaroptera brachyura, Chlorocichla falkensteini, Cyanomitra obscura, Cyanomitra verticalis, Dryoscopus senegalensis, Ploceus nigerrimus, Ploceus nigricollis, Psalidoprocne pristoptera, Psalidoprocne fuliginosa, Tchagra australis, Terpsiphone batesi and Turdus pelios, and six non-passerine members, Ceyx pictus, Colius striatus, Pogoniulus bilineatus, Trachylaemus pupuratus, Turtur afer and Turtur tympanistria (Table 3). Always according to Dajoz26 our analyses show clearly that the major part of species (47.06%) sampled were simply rare (Table 3).
Scientific name |
Frequency |
Common name |
Occurrence (%) |
Absolute abundance |
Relative abundance (%) |
Acrocephalus baeticatus |
1 |
African Reed-warbler |
12.5 |
1 |
0.63 |
Amblyospiza albifrons |
2 |
Thick-billed Weaver |
25 |
2 |
1.27 |
Bradornis fuliginosus |
1 |
Sooty Flycatcher |
12.5 |
3 |
1.9 |
Camaroptera brachyura |
2 |
Green-backed Camaroptera |
25 |
2 |
1.27 |
Campethera cailliautii |
1 |
Little Spooted Woodpecker |
12.5 |
1 |
0.63 |
Chalcomitra adelberti |
1 |
Buff-throated Sunbird |
12.5 |
1 |
0.63 |
Chlorocichla falkeinsteini |
3 |
Yellow-necked Greenbul |
37.5 |
3 |
1.9 |
Chlorocichla simplex |
5 |
Simple Greenbul |
62.5 |
7 |
4.43 |
Chrysococcyx cupreus |
1 |
African Emerald Cuckoo |
12.5 |
1 |
0.63 |
Chrysococcyx klaas |
1 |
Klaas’s Cuckoo |
12.5 |
1 |
0.63 |
Cinnyris batesi |
1 |
Bates’s Sunbird |
12.5 |
1 |
0.63 |
Cinnyris chloropygius |
1 |
Olive-bellied Sunbird |
12.5 |
2 |
1.27 |
Cinnyris minullus |
1 |
Tiny Sunbird |
12.5 |
1 |
0.63 |
Cinnyris superbus |
4 |
Superb Sunbird |
50 |
6 |
3.8 |
Colius striatus |
2 |
Speckled Mousebird |
25 |
3 |
1.9 |
Cyanomitra obscura |
3 |
Western Olive Sunbird |
37.5 |
4 |
2.53 |
Cyanomitra verticalis |
3 |
Green-headed Sunbird |
37.5 |
5 |
3.16 |
Dryoscopus senegalensis |
3 |
Red-eyed Puffback |
37.5 |
3 |
1.9 |
Estrilda melpoda |
1 |
Orange-cheeked Waxbill |
12.5 |
1 |
0.63 |
Eurillas latirostris |
5 |
Yellow-whiskered Greenbul |
62.5 |
8 |
5.06 |
Eurillas virens |
2 |
Little Greenbul |
25 |
5 |
3.16 |
Gymnobucco bonapartei |
5 |
Grey-throated Barbet |
62.5 |
13 |
8.23 |
Halcyon senegalensis |
1 |
Woodland Kingfisher |
12.5 |
1 |
0.63 |
Hedydipna collaris |
1 |
Collared Sunbird |
12.5 |
1 |
0.63 |
Hylia prasina |
1 |
Green Hylia |
12.5 |
2 |
1.27 |
Ispidina picta |
2 |
African Pygmy-kingfisher |
25 |
2 |
1.27 |
Laniarius luehderi |
1 |
Luehder’s Bushrike |
12.5 |
1 |
0.63 |
Lonchura bicolor |
1 |
Black-and-white Mannikin |
12.5 |
1 |
0.63 |
Mandingoa nitidula |
1 |
Green-backed Twinspot |
12.5 |
1 |
0.63 |
Nicator vireo |
1 |
Yellow-throated Nicator |
12.5 |
1 |
0.63 |
Ochrospiza mozambica |
1 |
Yellow-fronted Canary |
12.5 |
1 |
0.63 |
Platysteira cyanea |
1 |
Brown-throated Wattle-eye |
12.5 |
1 |
0.63 |
Ploceus cucullatus |
5 |
Village Weaver |
62.5 |
8 |
5.06 |
Ploceus nigerrimus |
2 |
Viellot’s Black Weaver |
25 |
2 |
1.27 |
Ploceus nigricollis |
2 |
Black-necked Weaver |
25 |
3 |
1.9 |
Pogoniulus atroflavus |
1 |
Red-rumped Tinkerbird |
12.5 |
1 |
0.63 |
Pogoniulus bilineatus |
2 |
Yellow-rumped Tinkerbird |
25 |
3 |
1.9 |
Psalidoprocne fuliginosa |
2 |
Mountain Sawwing |
25 |
7 |
4.43 |
Psalidoprocne pristoptera |
3 |
Black Saw-wing |
37.5 |
4 |
2.53 |
Ptyonoprogne fuligula |
1 |
Large Martin Rock |
12.5 |
2 |
1.27 |
Pycnonotus barbatus |
4 |
Common Bulbul |
50 |
11 |
6.96 |
Spermophaga haematina |
4 |
Western Bluebill |
50 |
6 |
3.8 |
Tchagra australis |
2 |
Brown-crowned Tchagra |
25 |
3 |
1.9 |
Terpsiphone batesi |
2 |
Bates’s Paradise-flycatcher |
25 |
2 |
1.27 |
Terpsiphone rufocinerea |
1 |
Rufous-vented Paradise-Flycatcher |
12.5 |
1 |
0.63 |
Terpsiphone viridis |
1 |
African Paradise-Flycatcher |
12.5 |
1 |
0.63 |
Thescelocichla leucopleura |
1 |
Swamp Palm Bulbul |
12.5 |
1 |
0.63 |
Trachylaemus purpuratus |
3 |
Eastern Yellow-billed Barbet |
37.5 |
3 |
1.9 |
Turdus pelios |
3 |
African Thrush |
37.5 |
5 |
3.16 |
Turtur afer |
3 |
Blue-spotted Wood Dove |
37.5 |
6 |
3.8 |
Turtur tympanistria |
3 |
Tambourine Dove |
37.5 |
3 |
1.9 |
Total |
|
|
|
158 |
100 |
Table 3 Specific abundance and occurrence of each captured species of the Eloumden avifauna during the bird survey between September 2015 and February 2016 in the relictual Eloumden mountain of the Congo Basin Forest (Central Africa)
Diversity indexes
The Shannon index was of 2.56 (familial level), 3.21 (generic level) and 3.62 (specific level) (Table 4). Concerning the Simpson index, values were of 0.90 (familial level), 0.95 (generic level) and 0.96 (specific level) (Table 4). As for the Shannon index, the general tendency is the same than the Simpson index with high values (Table 4). The Equitability index values were of 0.85 (familial level), 0.89 (generic level) and 0.92 (specific level) (Table 4); we noted that these last values appear far from 0 (Table 4).
Taxinomic level |
Family |
Generic |
Specific |
Taxa S |
19 |
37 |
51 |
Individuals |
158 |
158 |
158 |
Dominance_D |
0.11 |
0.05 |
0.04 |
Simpson_1-D |
0.89 |
0.95 |
0.96 |
Shannon_H |
2.48 |
3.21 |
3.62 |
Equitability_J |
0.84 |
0.89 |
0.92 |
Table 4 Diversity indexes of the Eloumden avifauna within their life environment obtained from the PAST software25
Avifauna of the Eloumden mountain, a mount of the congo basin forest
The biodiversity of the Congo Basin Forest is of world global importance because of the species richness but also because this Forest harbors the most diverse assemblage of plants and animals in Africa including more than 1000 bird species.1 In Cameroon, more than 970 bird species (resident, passage migrant, intra-African migrant, Palearctic migrant, visitor and vagrant) are recorded and especially according to surveys carried out, representatives of the passerines are always more numerous.2–4,6,7,10 As for previous surveys, the Eloumden mountain survey confirms the high representativeness of the passerines compared with the non-passerines (Table 1). More generally in line with the Centre region of Cameroon, some non-passerine families such as Lybiidae, Columbidae, Cuculidae, Coliidae, Picidae and Alcedinidae have been already mentioned and several passerine families among them Pycnonotidae, Sylviidae, Ploceidae, Estrildidae, Fringillidae, Hirundinidae, Nectariniidae, Monarchidae, Malaconotidae, Muscicapidae, Platysteiridae, Turdidae have been pointed out but until now most of these non-passerine and passerine families are not directly mentioned in relation with the relictual Eloumden mountain.3,4 Newly we found species’ representatives of the non-passerine families (Lybiidae) and passerine families (Hirundinidae, Muscicapidae, Nectariniidae, Nicatoridae) in the Centre region of Cameroon (Tables 1&3). According to our results, one new genus, Bradornis, and seven new species, until now not recorded in this mountain of the Congo Basin Forest, have been identified. It’s about: Trachyphonus purpuratus Verreaux & Verreaux, 1851 belongs to the Lybiidae’s family27 (Figure 4): this species has an extremely large range but until now, it is not recorded in this region. This pretty barbet has a large repertoire and has been caught in November 2015 during the breeding season; it is a common forest resident and sedentary bird.28,29
Psalidoprocne fuliginosa Shelley, 1887 belongs to the Hirundinidae’s family27 (Figure 5): until now recorded only in Cameroon mountain and Bioko, this swallow is known for its seasonal movements.28 According to del Hoyo et al.,29 it is probably a resident but some records suggested possible post-breeding movements. We have just caught some specimens in another mountain of this region (Febe mountain).
Ptyonoprogne fuligula Lichtenstein, 1842 belongs to the Hirundinidae’s family27 (Figure 6): this small bird has an extremely large range from Northern to Southern Africa but until now, it is not formally recorded in this region. Sometimes described as a common resident, this swallow has been recorded as a vagrant bird in Gabon.30 We caught it in December 2015 during its breeding season.
Bradornis fuliginosus Cassin, 1855 belongs to the Muscicapidae’family27 (Figure 7): this species has an extremely large range but it has not been recorded by Germain et al.,3 and Louette4 it is an uncommon or local common forest resident throughout range but often possibly and locally abundant.29
Cinnyris batesi Ogilvie-Grant, 1908 belongs to the Nectariniidae’s family27 (Figure 8): restricted in some countries of the Gulf of Guinea but also found from eastern Liberia, Ivory Coast and Ghana, this species is a rare or local common forest resident.28 In Cameroon, this sunbird is especially abundant in the South and until now, it was not recorded in this area.3,4
Chalcomitra adelberti Gervais, 1833 belongs to the Nectariniidae’s family27 (Figure 9): until now recorded from Sierra Leone to South East of Nigeria but also in west of Cameroon, this species is never recorded in this region;3,4,28 it is a general uncommon or local common forest resident throughout range.29
Acrocephalus baeticatus Vieillot, 1817 belongs to the Acrocephalidae’s family27 (Figure 10): this species has a large range but it is patchily distributed. In Cameroon, this African reed warbler was restricted in North-West and South of Cameroon and until now, it was not recorded in this relictual area.28 Only, it is an intra-African migrant.28 Concerning its song, we noted that it is a rhythmic series of repeated squeaky notes (Billy Nguembock personal observation).
Distribution and diversity of the avifauna in the Eloumden mountain
Based on statistical analyses, this mountain of Congo Rainforest shows high avifaunistic diversity from base to tip (Table 4). Our results show an absence of supremacy of one species (H’ = 3.62; Table 4); therefore, species have practically the same abundance. Generally, ecological biogeographers explain patterns of variation in the distribution of the species using environmental factors31,32 but other authors such as Hawkins et al.,33 and Fjeldsa et al.,34 tried to link the interaction between evolutionary and environmental factors. Concerning the relictual Eloumden mountain, firstly in this mountain, the vegetation appears uniform from the base to tip with by place the primary forest and sometimes the secondary forest intermixed with scrub land.35 Secondly in the Eloumden avifauna, we found a mixture of poor dispersers (specialists) such as Nectariniidae, etc. and better dispersers (generalists) such as Fringillidae, etc or even migratory birds such as Acrcephalus baeticatus, etc.; this mixture of taxa should be thus essentially due to the presence of luxuriant vegetation all year round. In fact, the particularity of the Eloumden mountain with the hillside in front of the wind is that it offers favourable conditions for birds in this ecosystem.12 Louette4 revealed that the distribution of some Cameroonian birds seems to be directly related to the vegetation; the distribution within this mountain corroborates this principle with the obtained Equitability index (J’= 0.92 species, Table 4) which suggests an equal distribution of individuals in this environment. As for the two first index values, the Simpson diversity index obtained of 0.96 goes in the same way (Table 4); this value supports a high avifaunistic diversity and especially confirms the tendency of an absence of supremacy of one species which could be explained by lush vegetation from the base to the summit. Thus as Louette4 our study suggests that the pattern of variation in distribution of birds in the Eloumden mountain appears to lean on environmental factors and particularly the vegetation.
Correlation between species variety and availability of food in the Eloumden mountain
Based on captured individuals, the Pycnonotidae family appeared the most abundant, followed by the Nectarinidae family as well as the Lybiidae family (Figure 2 & Table 1). Always according to our results, the Nectariniidae family appeared the most diversified, followed by the Pycnonotidae family (Figure 3). Thus concerning passerine birds within the abundant Pycnonotidae family, there is genera Chlorocichla (C. simplex with FO=62.5%) and Eurillas (E. latirostris with FO=62.5%) while within the abundant Nectariniidae family, there is two genera (Cinnyris and Cyanomitra) with a lot of species which had a weak frequency of occurrence (Table 3). For non-passerine birds, only one genus Gymnobucco (G. bonapartei with FO=62.5%) appeared abundant (Table 3). Globally, abundantly captured birds are forest species, although they can be found in other biotopes, which clearly shows that the study site is actually primary forest, although it appears to be degraded to some places.12 Thus for members of Pycnonotidae caught, Eurillas latirostris and Chlorocihla simplex eat almost essentially fruits and berries and sometimes they glean insects from the vegetation.28,29 Representatives of Cyanomitra and Cinnyris, Cyanomitra verticalis mainly eats small fruits and seeds, oil-palm sap as well as nectar from wide variety of flowers while Cinnyris superbus eats nectar from several flowers, seeds including those of Xylopia aethiopica, fruits but also insects, spiders and snails.28,29 Cyanomitra obscura eats insects, spiders, nectar, pollen, small berries, seeds, fruits and banana pulp.29 Concerning the African barbet Gymnobucco bonapartei, it eats essentially diverse fruits and berries such as figs and those of Allophyllus, Ficus, Musanga, etc.28,29In their study, Rajpar et al.,36 revealed that the habitat characteristics such as vegetation composition, vegetation structures and microclimate variables are the key factors that influenced the distribution, diversity and density of the wetland bird species. But more specifically in their recent study, Girma et al.,37 noted a high correlation between the availability of food and cover in a given environment and the abundance of bird species; according to these authors37 and Kiros et al.,38 the abundance of bird species is directly affected by the availability of food sources in an ecosystem which is mainly influenced by vegetation composition and structure. We note that the structure as well as the composition of the Eloumden vegetation are particularly influenced by the hillside in front of the wind which receives lots of sun and precipitations throughout the year and constitutes an abundant food source for many birds,12 always according to the same author,12 in some places, human activities, especially some work field, have significantly altered the vegetation with fruit trees and vegetables. Thus the vegetation of the Eloumden mountain offers a food source for many birds among them seeds, fruits, berries, nectar from diverse flowers as well as fruit trees and vegetables, and this would certainly explain the species variety caught in this mountain Eloumden of the Congo Basin Forest.
This current study strongly confirms that the structure as well as the composition of vegetation play a main role in the species diversity at least at the local level. Indeed, the vegetation of the Eloumden mountain which receive lots of sun and precipitations throughout the year offers, through the permanent growth of plants, a food sources for many birds among them seeds, fruits, berries, nectar from diverse flowers as well as fruit trees and vegetables. Otherwise based on our diversity and distribution analyses, the pattern of variation in distribution of birds in the Eloumden mountain appears to lean on environmental factors and, once again in this case the vegetation which played the main role with a bird mixture of poor and better dispersers, migratory and vagrant due to presence of luxuriant vegetation all year round and this would explain the lesser competition noted (D = 0.96).
This work was conducted with the permission of the chief of the Eloumden I village, his majesty BOMBA, who permitted us to carry out this study. We wish to thank the guide, Mr. Aimé, for all his efforts and his good knowledge of the study site which facilitated birds capture. We also wish to thank Miss BIDJA Bernadette for her help during this study. We thank the Zoology Laboratory of the University of Yaounde I especially the ornithological team for the technical material which has been used for the realization of this scientific work. We also thank all persons especially Mr. MBOG Jacques who have read this paper to ameliorate its quality through his scientific comments.
Authors declare that there is no conflict of interest.

©2019 Nguembock, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.