Advances in
eISSN: 2572-8490


Research Article Volume 2 Issue 1
1Department of Pathology and Laboratory Medicine, Rutgers, Robert Wood Johnson Medical School, USA
2OptoVibronex, LLC, Mt. Bethel, PA , USA
3Graduate Program in Biomedical Engineering, Rutgers, The State University of New Jersey, USA
Correspondence: Frederick H Silver, Department of Pathology and Laboratory Medicine, Robert Wood Johnson Medical School, Rutgers, the State University of New Jersey, 675 Hoes Lane, Piscataway, NJ 08854, USA
Received: February 01, 2017 | Published: February 20, 2017
Citation: Silver FH, Silver LL, Shah R. Viscoelastic behavior of allografts and scaffolds composed of extracellular matrices. Adv Tissue Eng Regen Med Open Access. 2017;2(1):114-119. DOI: 10.15406/atroa.2017.02.00019
Collagen is the major structural fiber found in mammalian tissues. It is a protein in the form of a triple-helix that is found in several subfamilies, the most abundant of which is the fiber-forming group containing Types I, II and III. Type I collagen is found in tendons, skin, cornea, bone, lung and vessel walls. This collagen is thought to give rise to the high tensile strengths of collagen fibers in tissues; in addition, it is actively involved in other physiologic processes such mechanotransduction. However, the non-linear mechanical behavior and viscoelasticity of collagen fibers make analysis of the mechanical properties of tissues complicated.
Mechanistically, during mechanical loading, a tensional increase in the D period is observed with increasing strain that is associated with:
The state of mechanical loading of tissues dictates the regulation of tissue metabolism by up- or down-regulating mechanotransduction through several mechanisms including the phosphorelay pathways.
In this paper, we discuss the relationship between collagen hierarchical structure and its viscoelastic mechanical properties. Using vibrational analysis and optical coherence tomography it is hoped that the mechanical properties of collagenous tissues can be studied in vivo in order to better understand tissue mechanics and to be better able to offer early diagnosis and differentiation of different disease states.
Keywords: collagen fibers, mechanical properties, optical coherence tomography, vibrational analysis, tendon, skin, cartilage, elastic arteries
ECM, extracellular matrix; OCT, optical coherence tomography
Collagen fibers form the basic structural components of the extracellular matrix (ECM) of vertebrates that serve to: store elastic energy during muscular deformation, transmit stored energy into joint movement, and transfer excess energy from the joint back to the attached muscles for dissipation.1 They also act as mechanotransducers by transferring stress borne by the musculoskeleton to the attached cells in order to regulate tissue metabolism, either up- or down, as a result of changes in mechanical loading.2 The viscoelastic behavior of ECM is very important in understanding the tissue reaction to implanted allografts and tissue engineered materials. It is known that excessive stress at the implant-tissue interface leads to intimal hyperplasia with vascular grafts as well as fat tissue necrosis associated with the pressure produced with breast implants and fat necrosis at the femoral catheter site after use of pressure to achieve closure.3
For these reasons collagen fiber mechanical behavior in ECMs is intimately related to tissue reactions at the implant-tissue interface. However, analysis of the basic mechanisms behind this behavior is complicated by the viscoelasticity of collagenous tissues, the presence of other components besides collagen, and mechanotransduction processes. The viscoelasticity of collagenous tissues leads to non-linear behavior; however, recent studies have identified methods to analyze this behavior.2,4–6 In this paper, we will review the relationship between ECM molecular and hierarchical structures, mechanical properties and mechanotransduction to better understand events that occur at the implant-host tissue interface.
Collagen molecular structure
Collagen is a protein in the form of a triple-helix that is found in several subfamilies the most abundant of which is the fiber-forming group containing Types I, II and III. Types I, II, and III form the basic structural units of collagen fibrils and fibers along with some minor types of collagen including types V, IX and XII.7 Type I collagen is found in tendons, skin, cornea, bone, lung and vessel walls.8 This collagen is thought to give rise to the high tensile strengths of collagen fibers in tissues; in addition, it actively is involved in other physiologic processes such mechanotransduction through integrin dependent activation of the phosphorelay pathways.5 The activation of the phosphorelay pathways by changes in mechanical stresses on collagen fibers lead to up- or down regulation of protein synthesis and cell division that modulates the events that occur at the implant-tissue interface. Viscoelasticity and mechanotransduction have been studied extensively in tissues such as skin and tendon and therefore we will explore these properties further.
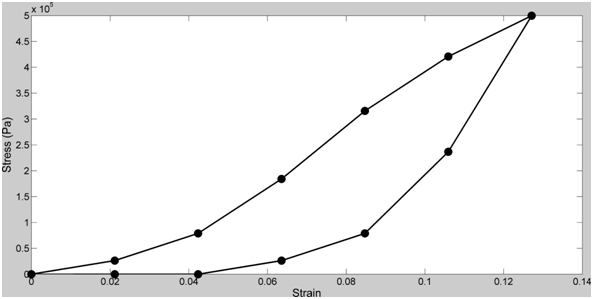
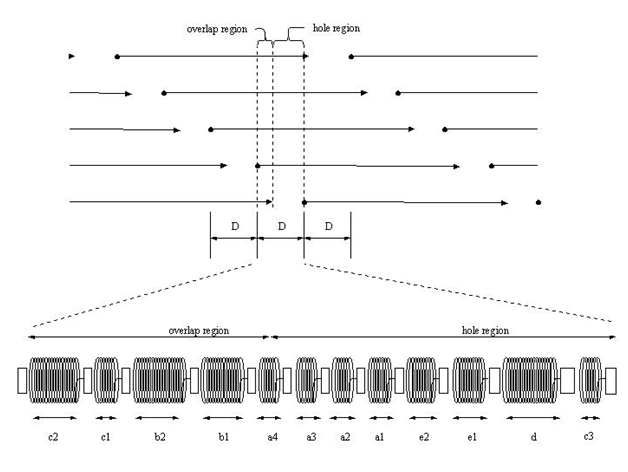
Tendons and skin have been studied in vitro and exhibit non-linear viscoelastic stress-strain behaviors (Figure 1).9–12 The non-linear behavior is derived from the structural hierarchy of these tissues and the domain structure of the collagen triple helix (Figure 2). Viscoelasticity is related to the molecular and fibrillar deformation patterns of collagen fibrils and fibers as discussed below.
Collagen, when stained with heavy metals, in tissues is recognized by transmission microscopy by its regular repeat pattern of the charged amino acid residues. In the quarter-staggered packing pattern, the amino acid sequence of five molecules in cross-section is repeated every 64nm, a distance termed the D period (Figure 2). The D period varies from about 64 to 67nm depending on the tissue of origin. In tendon, the D period is about 64nm and in skin it is about 67nm. Collagen microfibrils in tissues are packed into a quasi-hexagonal array that forms fibrils and fascicles in tendon.
Collagen crosslinking
In fibril forming collagens, the ability to store, transmit and dissipate energy requires crosslink formation between molecules within a microfibril and between microfibrils and other structural units. These crosslinks include lysine and hydroxylysine derivatives and other amino acid residues including histidine that are at the ends of the molecules (Figure 2).4 Additional crosslinking also occurs during aging and involves glucose molecules.7 The stiffening and poor energy dissipation of collagen fibers associated with aging of the skin involves collagen fiber fragmentation by exposure to UV light and glucose derived crosslinking. In the absence of crosslinking, the mechanical behavior of collagenous materials is primarily viscous with little energy storage.3
Viscoelastic behavior of collagen fibers
Viscoelasticity of collagenous tissues may be important in resisting impact loads especially in the musculoskeleton, however, it complicates the understanding of ECM behavior since most real-time measurements made on these tissues contain both elastic and viscous contributions.11 The elastic behavior varies from as high as about 75% of the total stress for tendon to as low as about 50% for skin depending on the collagen fiber orientation, rate of loading, degree of crosslinking and the quantities of other tissue constituents.11
A number of excellent studies have been published that have helped in the interpretation of the viscoelastic behavior of tendon and other tissues at the molecular and fibrillar levels. Much of our current understanding of the relationship between hierarchical structure and viscoelastic behavior of ECMs is based on studies of the mechanical properties of developing and mature tendons.13–16 The collagen fibril length appears to be important for energy storage and for viscous energy loss due to molecular and fibrillar deformation during tensile deformation.
Mechanistically, during mechanical loading, a tensional increase in the D period is observed with increasing strain that is associated with:
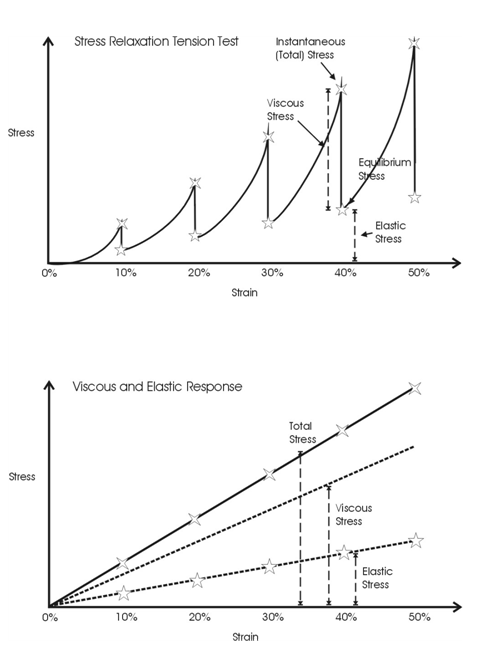
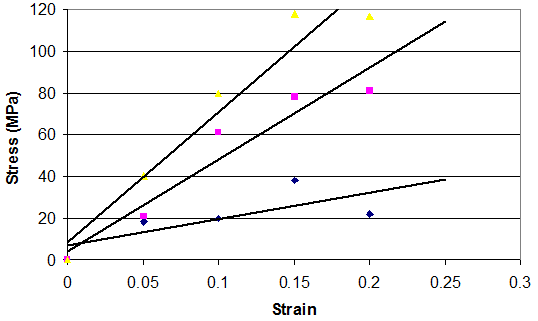
The time-dependent behavior of collagenous materials makes it difficult to interpret stress-strain relationships for these tissues. However using incremental stress-strain curves, the elastic and viscous behaviors can be separated and analyzed in terms of tissue structure.11 The viscoelastic properties of ECMs have been obtained by constructing incremental stress-strain curves for a variety of tissues including tendon2,11 (Figure 3). Such incremental stress-strain curves are derived for tendon and other ECMs by stretching the tissue in a series of strain increments and then allowing the stress to relax to an equilibrium value at each strain increment before another strain increment is added (Figure 3 top).11 By subtracting the elastic stress (equilibrium stress value) from the initial or total stress value, the viscous stress is obtained. By plotting the equilibrium stress versus strain and the total stress minus the equilibrium stress versus strain (Figure 3bottom) we get elastic and viscous stress-strain curves for tendon.2 (Figure 3). From these curves and the literature, important information can be obtained concerning the mechanism of stretching and sliding of the collagen molecules and fibrils that make up the structure of tendon fibers.2 Note in the absence of the crimp the relationship between elastic stress and strain is approximately linear (Figure 4). It turns out that the slope of the elastic stress-strain curve is proportional to the elastic modulus of the collagen molecule,2,19 while the viscous stress at a particular strain is a measure of the fibril length.2,19 The problem with conducting true incremental stress-strain testing on collagenous materials is that it takes up to 24 hours for the stress to relax to an equilibrium value, before another strain increment can be added. However, the value of doing such an experiment is that it has been reported that the elastic modulus is strain-rate independent for a variety of collagenous tissues and self-assembled collagen fibers.2 However, construction of an incremental stress-strain curve is a very tedious task and other methods need to be developed to obtain the elastic modulus for collagenous materials both in vitro and in vivo.
Non-destructive methods for studying viscoelasticity of collagen tissues and scaffolds
The ability to monitor the mechanical properties of ECMs and implants in vivo is an important measurement needed for early diagnosis of disease, the ability to follow disease progression and to evaluate healing after implantation of a medical device.12 Physicians daily “palpate” changes in the properties of tissues associated with tumors and calcification suggesting that there are major changes in the structure and properties of collagen and ECMs during disease processes. It is essential that clinicians be able to assess the changes at the collagen fibril and fiber levels of structure to accurately diagnose and treat diseases such as cancer as well as to follow the wound healing responses to tissue engineered materials and implants.
The use of vibrational analysis in concert with OCT allows one to measure the resonant frequency and mechanical properties of ECMs and implants non-destructively and non-invasively (Figure 5) (Figure 6) at different strains.6,12 Since the measurements at different strains using vibrational analysis yield different resonant frequencies, this data can be used to calculate the elastic modulus from incremental testing of ECMs and implants relatively quickly. It is important to match implant mechanical properties with that of the host tissue to reduce the degree of up-regulation of mechanotransduction. Up-regulation of mechanotransduction may lead to increases in capsule formation and tissue hyperplasia at the tissue-implant interface.
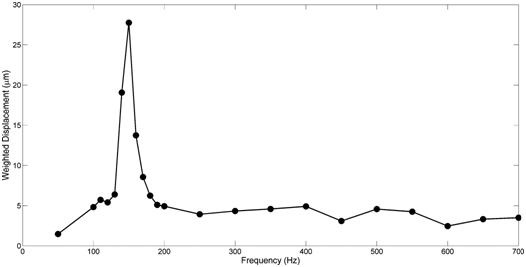
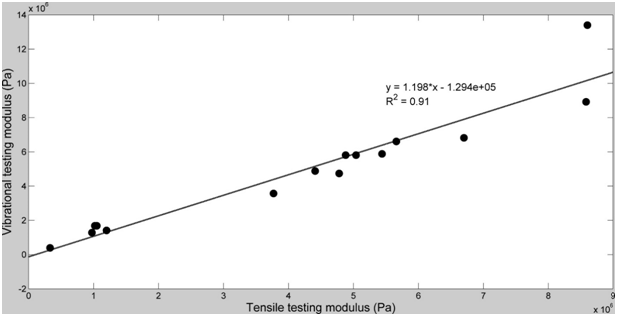
Of primary concern besides matching the elastic behavior of ECMs and implants, is that any excess energy imparted at the interface has to be either transmitted away from the interface or dissipated as heat. While the energy that goes into elastic behavior can be transduced into new protein synthesis and cell division, the viscous portion of the energy can only be dissipated as heat or mechanical movement at the tissue-implant interface.
Mechanotransduction in collagenous tissues
Collagen fibrils are a major factor in the conversion of mechanical forces and work into stored energy; in many cases, this energy is stored in the form of high molecular weight polymers such as collagen fibers.2 When muscles and tendons are loaded, the muscle does work on the collagen fibers in tendon and energy is stored as work in the tendon. During tendon stretching, the applied force results in work stored elastically, by stretching the flexible regions of the triple helix. This energy is released after the load is removed and transmitted to the muscle or the energy is stored in the form of high molecular weight proteins via a process termed mechanotransduction.
Collagen fibrils are attached to cell membranes through attachment molecules such as integrins and other cell surface macromolecules. During stretching of the collagen fibers, tension is transmitted through the cell membrane setting into motion the activation of the phosphorelay pathways inside the cell.2 Tension typically activates the MAP kinase pathways leading to synthesis of new collagen to “bolster” the ability of the tendons and other tissues to support loads.2 Thus, some of the mechanical energy generated from the muscle tension is stored in the form of high molecular weight polymers; energy is stored in the form of covalent bonds that link amino acids together in the newly synthesized collagen fibers. When external tensile loads decrease, the collagen fibers are resorbed and energy is released from the broken covalent bonds. This can occur during non-loading events, such as prolonged bed rest or when an astronaut is in a reduced gravitational field for a prolonged time period.
In this manner, collagen fibers are dynamic structures that are constantly growing or resorbing depending upon the level of tension they experience. The interactions between the collagen fibers and cells in tissues are an exciting part of the dynamics that occur in ECM biology and ultimately affect health and the pathogenesis of disease processes such as cancer.
Recent advances in the understanding of integrin mediated mechanotransduction20,21 are important to understand the relationship between external mechanical loading and the events that occur at the interface between the ECM, surrounding cells and any implant that is in contact with the cell-ECM interface. This suggests that any interruption of integrin mediated binding to the surrounding ECM at the implant-tissue interface has consequences in terms of modifying mechanotransduction and the cellular response to allografts and implants.
Integrin mediated mechanotransduction plays an important role in development and tissue homeostasis.20 After implantation of an allograft or tissue engineered scaffold, cell binding occurs. ECM receptors such as integrins facilitate assembly of different ECM components into a variety of geometrical form.22 Specifically integrin α and β subunits bind to type I collagen receptors5 and lead to a conformational change in the integrin subunits (Figure 7). Integrins then recruit numerous proteins to their cytoplasmic tails forming nasant adhesions some of which stabilize and form focal adhesions. Focal adhesion maturation requires further integrin clustering, intracellular F-actin binding and reinforcement of linkages between actin and actomyosin. Activated integrins are coupled to F-actin through actin binding proteins including talin and vinculin, These molecules are involved in the “molecular clutch” that is responsible for cell movement as a result of actin flow inside the cell as F-actin is polymerized leading to force generation and propulsion of the cell body.20
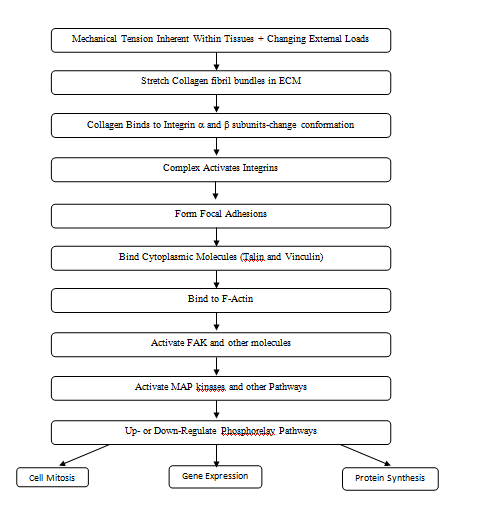
Phosphorelay pathways can be activated not only by integrin binding to ECMs, but by direct stretching of the following: cell membranes, ion channels, intracellular junctions, and growth factor and hormone receptors. Activation of these moieties leads to changes in protein synthesis, altered gene expression and cell mitosis.19 (Figure 7). All these events occur at the cell-allograft and cell-tissue engineered material interface and dictate rate, composition and extent of tissue in growth. Providing the correct boundary forces and stresses to equilibrate the implant-tissue interface is important to prevent cellular hyperplasia and excessive collagen deposition.
Collagen fibers are the structural elements found in vertebrate tissues that transmit forces, store and dissipate energy. Collagen fibers limit the deformation of tendon and other load bearing tissues and have a hierarchical structure that includes collagen molecules, microfibrils, fibrils, fibers and fascicles. Collagen molecules are packed into a quarter-stagger packing pattern with neighboring molecules staggered by multiples of D, which is about 22% of the molecular length. During mechanical deformation, collagen molecules are stretched as well as the gap region of the D period. The elastic modulus of collagen molecules can be calculated from the slope of the tensile stress-strain curve after correction for, viscous slippage, the collagen content and the change in axial rise per amino acid residue that occurs during tensile deformation. In tissues such as tendon, the low modulus region occurs due to unfolding of the crimped collagen fibers. The differences in the values of the modulus measured reflect differences in collagen concentration, orientation and the presence of other materials. The low modulus region involves geometric alignment of collagen fibers with the tensile axis as well as stretching of other components in series with the collagen fibers. At larger strains, collagen molecules and fibrils slide by each other, which lead to energy losses and viscous effects.
Mechanical loads applied externally to collagen fibers in ECMs, integrin sub units and other binding elements play important roles in mechanotransduction and events that occur at the implant-host interface (Figure 7). Excessive stress at the interface can lead to intimal hyperplasia and excessive ECM deposition ultimately leading to implant failure or encapsulation. It is hoped that measurement of the mechanical properties of tissues and implants in vivo will help to better understand collagen fiber behavior in health and disease and to prevent stress concentrations at the interface between host tissues, allografts and tissue-engineered materials.
None.
The author declares no conflict of interest.

©2017 Silver, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.