Advances in
eISSN: 2378-3168


Research Article Volume 14 Issue 1
Department of Zoology, Tribhuvan University, Amrit Campus, Nepal
Correspondence: Shyam Narayan Labh, Department of Zoology, Tribhuvan University, Amrit Campus, Nepal
Received: January 10, 2024 | Published: February 29, 2024
Citation: Labh SN. Vitamin C (L-ascorbate 2-triphosphate Calcium) enhances the growth, immunobiochemical, and haemato-morphological performance of common carp Cyprinus carpio. Adv Obes Weight Manag Control. 2024;14(1):8-18. DOI: 10.15406/aowmc.2024.14.00406
Many people have a cultural practice of eating fish, and it has many health benefits. It is a good source of protein, fatty acids, vitamins, minerals, and other micronutrients. Fish has an outstanding nutritional profile and carps lack l-gulonolactone oxidase (EC 1.1.3.8), they are unable to generate vitamin C, which is necessary for fish growth from an exogenous source. In order to assess the effects of vitamin C, as l-ascorbate-2-triphosphate Calcium (LATP-Ca), on the physiology and growth, a 50-day feeding experiment was carried out on common carp (Cyprinus carpio). For six weeks, common carp (59 ± 0.56g) were kept in triplicate cemented tanks on four semi-purified diets (treatments A, B, C, and D, respectively) supplemented with 0, 1000, 1500, and 2000 mg/kg vitamin C, l-ascorbate-2-triphosphate Calcium (LATP-Ca). Weight gain and weight gain (%), SGR, and FCR were assessed for growth study; quantification of vitamin C in the kidney, liver, brain, and muscles was examined, and then serum glucose, triglycerides, creatinine, and urea were examined for biochemical performances and total serum protein, albumin, and globulin as well as liver function tests such as SGOT, SGPT, and ALP after full hematology was examined to understand the immune-haematological performances. It was observed that dietary vitamin C levels had a substantial (P < 0.05) impact on weight increase, weight gain %, specific growth rate, and tissue vitamin levels. While FCR, SGOT, SGPT, serum glucose, cholesterol, triglycerides, creatinine, and blood urea concentration level showed declining trends. Additionally, a complete blood hemogram profile and vitamin C were found to interact significantly, with the LATP-Ca treated group exhibiting improved performance. The effects of vitamin C on carp growth, physiology, and immune response are demonstrated by the treated group's superior blood cell morphological structure as compared to the control group. In conclusion, dietary vitamin C (LATP-Ca) at inclusion levels of 1500 to 2000 mg/kg enhanced carp development and shielded fish from growth, depression, and mortality.
Keywords: common carp, vitamin C, growth, enzyme, blood, tissues, immune responses
Aquaculture has become the fastest-growing sector among agriculture, and the allied food production sectors and Global Aquaculture Production was 114.5 million tonnes in 2018, comprising 82.1 million tons of aquatic animals, and 26 000 tonnes of ornamental seashells and pearls.1 Aquaculture is practiced by both the most impoverished farmers in developing countries and by multinational companies. Altogether 580 aquatic species are currently farmed all over the world, representing a wealth of genetic diversity both within and among species. Asia subsidized 85.6 percent to the increased output of 2.5 million tons of farmed aquatic animals in 2018 and looking forward to 2020 happening a "super-year" for fisheries and aquaculture, with a sequence of high-impact events, in the hopeful guess that the coronavirus pandemic improves soon.2
Carp larvae, like any other fish larvae, depend on natural food during the first few days of their life. In nursery conditions, high mortality and slow larval growth are common, and infectious diseases are the primary cause of economic loss in commercial aquaculture.3 High mortality, stunted growth rate, skeletal deformities, frequent disease outbreak are some of the common problems encountered in the nursery culture of carp.4 One possibility to reduce the incidence of high mortality is by ensuring adequate nourishment for critical nutrients like ascorbic acid, adequate feed distribution during the day to suit the rapid transit rates of the larva, and ensuring cleaner ambient water quality during larval raising. It should provide an improved immune status enabling the fish to resist stress and diseases.5 Therefore, the aquaculture industry began to emphasize the prevention of diseases rather than treatment with chemotherapeutics and antibiotics, which have criticized for their harmful side effects. Consequently, many studies have investigated the modulation of the fish immune system to prevent the outbreak of disease. Although vaccines promise the future prevention of diseases, vaccines are currently not effective against many commercially important diseases.6
Common carp (Cyprinus carpio) belongs to the order Cypriniformes and the family Cyprinidae, is the third most widely cultivated and commercially important freshwater fish species in the world and the native wild population of common carps is considered vulnerable to extinction by the International Union for Conservation of Nature (IUCN). However, it is widely distributed in almost all countries, prevalent in Asia and some European countries.7 In aquatic animal nutrition, vitamins are among the most expensive ingredients used in complete diet formulation and play essential roles in the organism, as cofactors for enzymes.8 An inadequate supply leads to reduced enzyme activities9 and, in turn, to both nonspecific responses such as weak growth, low survival, and increased susceptibility to infections and diseases as well as more specific deficiency signs and symptoms.10 Vitamin C (AA) is an essential micronutrient for healthy growth and the physiological function of most aquatic animals.11 Most fish species have a limited ability to synthesize vitamin C (Lightner et al. 1982), due to the absence of l-gulonolactone oxidase that is responsible for AA biosynthesis.12 So that signs of deficiency are observed when vitamin C is excluded from the diet. It has also been proposed to be potentially beneficial in increasing immune response and reducing oxidative damage to tissues.13
Vitamin C might be helpful for proper nutrient utilization because AA plays an essential role in certain aspects of protein metabolism14 and is an essential molecule in the overall health of animals.15 The findings of several studies indicated that dietary vitamin C could improve the growth performance and feed utilization for different fish species.16 The ascorbic acid (AA) is volatile in feeds because of the sensitivity to heat and light produced during the storage and processing of diets; however, more stable and bioavailable forms of AA are currently unavailable.17 Phosphate derivatives of AA, are more stable and bioavailable forms, currently available and shown to have antiscorbutic activity in several aquatic animal species,18 including L-ascorbyl-2-sulphate (C2S), L-ascorbyl-2- monophosphate-Mg (C2MP-Mg), L-ascorbyl-2-monophosphate-Ca (C2MP-Ca), L-ascorbyl-2-monophosphate-Na/Mg (C2MP-Na/Mg), L-ascorbyl-2-polyphosphate (C2PP), ascorbate-2-glucose (C2D) and L-ascorbate-2-triphosphate-Ca (LATP-Ca).19 The potential effects of several derivatives of A. A. have been investigated in several aquatic species such as C2S in rainbow trout, C2MP-Mg in Asian sea bass (Lates calcarifer), C2MP-Na in hybrid tilapia, C2PP in yellow croaker (Pseudosciaena crocea), C2MP-Ca in atlantic salmon (Salmo salar), C2-MP-Na/Ca in kuruma shrimp; and C2D in hybrid Clarias catfish (Clarias gariepinus).20 However, there is still limited evidence on the association between dietary A.A. derivatives and its antioxidant effects for fish species.21 A better understanding of modes of action may lead to practical and appropriate vitamin C in aquaculture as a stable form of phosphate derivatives. An experiment was conducted to understand the effects of vitamin C L-ascorbate 2-triphosphate-Ca (LATP-Ca) on growth, biochemical, immunological, and hematological and blood morphological of common carp during indoor culture system.
Preparation of experimental diets
Preparation of experimental diets supplemented with LATP-Ca (LATP-Ca) procured from Hi-Media Pvt, India and then four (A, B, C & D) experimental diets having crude protein (40%) were formulated containing graded levels (0, 1000, 1500, and 2000 mg A.A. kg -1 diets) of ascorbic acid (LATP-Ca) along with other ingredients viz. fishmeal, wheat flour, and cod liver oil (Table 1). All the ingredients were ground, milled, and mixed thoroughly to form a homogenous blend, then cod liver oil and water were added to form the dough. The prepared dough was passed through a feed maker using 2 mm die, and the pellet air-dried. The dried pellet further chopped into small pieces of required sizes of pellets and then conceded through a sieve to obtain the uniform size. Then, the diets were stored at -20°C until use.
|
Ingredients(g) |
Control |
T1 |
T2 |
T3 |
|
Fish meal |
58.24 |
58.24 |
58.24 |
58.24 |
|
Wheat flour |
36.76 |
35.76 |
35026 |
34.76 |
|
Fish oil |
5 |
5 |
5 |
5 |
|
Vit-C(LATP-Ca) |
0 |
1 |
1.5 |
2 |
|
Total |
100 |
100 |
100 |
100 |
Table 1 Preparation of experimental diets with LATP-Ca
Experimental fish and set-up
The juvenile common carp Cyprinus carpio used in this study were obtained from a commercial farm in Jahangirpuri, New Delhi, to the Department of Zoology, University of Delhi, New Delhi, India. Fish were acclimatized for one week under the aerated condition and fed with the basal diets (without LATP-Ca). Altogether 180 juvenile carps (59 ± 0.56g) divided into four groups (C, D-1, D-2, and D-3) were distributed into twelve rectangular cemented tanks (170 L) at the rate of 15 fish/tank following a completely randomized design. All triplicate groups of fish were fed two times daily at 9:00 h and 18:00 h. and the water quality parameters were monitored and maintained22 at the optimal level (temperature 27.9±0.12 to 31.6±0.030C; pH 7.5±0.07 to 7.9±0.11; D.O. 6.6±0.01 to 7.5±0.11 mg/l). Two thirds of the water was replenished at weekly intervals till the end of 90 days feeding trial.
Survival and growth performances
In this study, fish growth was assessed in terms of final weight gain (FWG), percentage Weight Gain (PWG), Specific Growth Ratio (SGR), and food conversion ratio (FCR) followed by percentage survival on regular monitoring up to the 96 days of feeding with LATP Ca:
FWG= Average final weight - Average initial weight
PWG = [Average final weight - Average initial weight] / initial weight] x 100
SGR (%/day) = [final body weight - initial body weight] × 100 /day).
FCR = Food intake / weight gain.
Survival %= Number of survived fish/initial number of fish x 100
(Note: All the fish weights in the top equations were calculated in gram unit)
Estimation of Vitamin C from tissue
Three fishes from each treatment were collected and anesthetized with tricaine methanesulfonate (MS-222) for 2-3 min. The liver, head-kidney, and body muscles were collected through proper dissection to assay the vitamin C (LATP-Ca) following Dabrowski and Hinterleitner method.23 Pre-weighed tissues were homogenized in ice-cold 250 mM perchloric acid (HClO4) containing 5% trichloroacetic acid (TCA) and 0.08% ethylenediaminetetraacetic acid (EDTA). The homogenate was centrifuged at 2700 x g for 20 min at 40C. Twenty-five microliter (25 µl) of 0.2% dichlorophenolindophenol (DCIP) was added to 250 µl of the deproteinized sample, and the mixture was incubated at 37°C for 1 h. Then 25 µl of 1% KBrO3 was added, and the mixture was incubated at 37°C for 1 h. After 1 h incubation at room temperature, 250 µl of 2% thiourea (in 5% meta-phosphoric acid) was added, followed by addition of an equal volume of 2% 2, 4-dinitrophenyl-hydrazine (DNPH) in 12 M H2SO4. All samples were incubated for 3 h at 60°C, then 0.5ml of ice-cold 18M H2SO4 was added to the samples. The sample was transferred into Eppendorf tubes and centrifuged at 11300 x g for 3 min. Absorbance was chronicled at 524 nm with a Milton Roy Spectronic 21D Spectrophotometer. The blank was prepared in the same manner, except that the buffer was used instead of the sample extract. Standard (20-200µg/ml) was prepared with vitamin C.
Collection of blood and immuno-biochemical parameters
The fish were numb with tricaine methane sulfonate (MS-222) at 250 ppm in water. The blood was drawn from the caudal vein using a heparinized disposable hypodermic needle and transferred into a disposable heparinized tube. Blood samples collected from six randomly selected fish from each treatment (1+1/replicate) at the termination of the feeding trial. Blood was drawn from one set of fish (three fish per replicate) used for serum collection and frozen in -20°C until use while another three-set used for hematological and morphological blood parameters. Thus, the collected blood serum used for following different biochemical studies.
Serum total protein profiles: Serum total protein of the fish sample was determined by Biuret method (Henry et al., 1974), albumin by BCG method24 using a laboratory diagnostic kit (Aspen Laboratories Pvt. Ltd., India). Albumin was subtracted from serum total protein to obtain globulin values, and the A/G ratio determined.
Serum metabolic profiles: For physiological performances, serum glucose, cholesterol, triglycerides, creatinine and blood urea were determined by using the standard kit.25
Serum enzyme profiles: IFCC kinetic method explained by Reitman and Frankel26 method used for the determination of serum glutamic oxaloacetic transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) while, and alkaline phosphatase (ALP) using kits (Bayer Diagnostics India Ltd., India).
Haemato-morphological parameters
Three fishes from each treatment were selected for blood samples (2. 5) to estimate the complete blood haemogram and blood morphology.
Complete blood haemogram: Fish hemoglobin (Hb) content was determined by the cyano-methemoglobin method27 using Drabkin's reagent. For erythrocyte count, the blood sample was diluted 1:200 in Hendricks' fluid, and cells counted on a Neubauer haemo-cytometer.28 Total leukocyte count determined following the method of Shahi et al.,29 Haematocrit was determined based on the sedimentation of blood, as described by Akinleye et al30. The Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) calculated from the results of Hb, RBC, and Hct as described by Dacie and Lewis.31
Blood morphology: Blood smears prepared according to Macedo et al32, total 1 ml blood sample collected, and a drop of blood smeared on a grease-free clean glass slide from each fish, and then thin blood film were prepared immediately after collection and stained with the combination of Wright's stain (Hi-Media) and Giemsa stain (Merck). First modified Wright's stain (Wright's stain: 4 ml, PBS: 2ml, Acetone: 3 ml and Distilled water: 31 ml) was poured on to the slide, kept for 10 min and then diluted with distilled water (pH 7.0-7.2). The sample further kept for another 20 min. The film was stained with diluted Giemsa (1:10) for 90 min. Finally, the slides were washed, air-dried, and observed under the microscope (Carl Zeiss Axioskop 40).
Statistical Analysis
In this study, all results for each parameter measured and expressed as mean ± standard error (Mn± SE). Data assessed using a one-way analysis of variance (ANOVA) with Aloe vera inclusion levels as a reason using the statistical package for the social sciences (SPSS) computer software (version 22). Duncan's multiple range tests at a significant level of 95% and used to determine significant differences between treatments. All data presented in the text, figures, and tables are means ± standard error, and P < 0.05 was considered as significant.
Survival and growth
The growth performance parameters and survival of the fish fed experimental diets presented in Table 2. Fish fed AA (LATP-Ca) based diets performed better than the control. A significantly higher FWG, PWG (33.2 % to 56.1%), and SGR (0.68 to 1.05) value recorded in the fish fed T3 diet (p<0.05). The improvement in growth tends to follow a linear direction as the level of dietary ascorbic acid (LATP-Ca) increased in the diets. A reverse trend observed in the case of FCR, with the lowest value recorded in the T3 fed group compared to the higher value in the control (Table 2). No mortality was recorded in all the groups throughout the experimental period.
|
Treatments |
FWG |
PWG |
SGR |
FCR |
S(%) |
|
Control |
62.80±0.35a |
33.2626±0.76a |
0.6836±0.01a |
2.52±0.05d |
100 |
|
T1 |
67.44±0.14b |
43.1006±0.31b |
0.8533±0.005b |
1.94±0.01c |
100 |
|
T2 |
69.15±0.23c |
46.7218±0.50c |
0.9128±0.008c |
1.79±0.01b |
100 |
|
T3 |
73.53±0.47d |
56.0153±1.01d |
1.0589±0.01d |
1.50±0.02a |
100 |
|
P-value |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
<0.05 |
Table 2 Growth performance and survival rate of Cyprinus carpio fed different experimental diets
Vitamin C from liver, head-kidney, and body muscles
The ascorbic acid (LATP-Ca) concentration in liver, head-kidney and body muscle of the experimental fish increased significantly (p<0.05) with increasing level of LATP-Ca in the diets. However, at higher supplementation above 1500 mg AA/kg, a decline in the tissue concentration of ascorbic acid was observed (Figure 1). The dietary level of LATP-Ca showed a second order polynomial relationship with tissue concentration in the liver (y = -0.0005x2 + 1.3559x + 327.12, R2 = 9774), muscle (y = -0.0002x2 + 0.6128x216.93, R2 = 0.932), and kidney (y = -0.0006x2 + 1.4576x + 392.38, R2 = 0.9482) (Figure 2, 3, & 4).
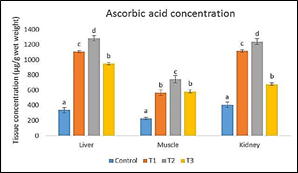
Figure 1 Ascorbic acid levels in the liver, muscle and kidney of C. carpio fed with different experimental diets.
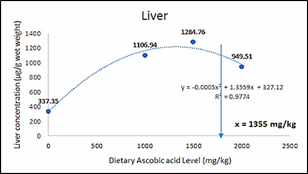
Figure 2 Second order polynomial of the relationship between dietary ascorbic acid and liver AA concentration of C. carpio.
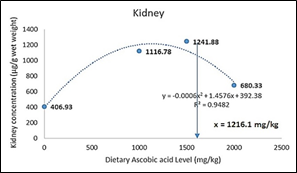
Figure 3 Second order polynomial of the relationship between dietary ascorbic acid and kidney AA concentration of C. carpio.
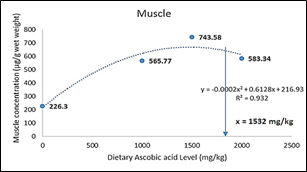
Figure 4 Second order polynomial of the relationship between dietary ascorbic acid and muscles AA concentration of C. carpio.
Immuno-biochemical performance
The serum total protein, albumin, globulin, and A/G ratio of different experimental groups are given in Table 3. Significantly higher total protein and albumin values were recorded in T3 fed group, which did not differ statistically (p>0.05) from the fish fed T2 diet. The globulin value showed no significant variation among the experimental groups. A/G ratio was highest in the T2 group, whereas the control group recorded a significantly (p<0.05) lowest value compared with the A.A. fed groups (Table 3). In the serum metabolic profiles, serum glucose, cholesterol, triglycerides, creatinine, and blood urea of different treated groups found a decreasing trend as compared to control (Table 4). Transaminases (SGOT & SGPT) and alkaline phosphatase (ALP) enzyme activities value are shown in Table 5. A statistically higher AST and ALT enzymes activities were observed in the control group and decrease with increasing attentiveness of ascorbic acid in the treatment groups, whereas ALP enzyme activities exhibited the opposite trend.
|
Treatments |
Total Protein |
Albumin(g/dl) |
Globulin(g/dl) |
A:G ratio |
|
Control |
2.57±0.9a |
0.90±0.01a |
1.67±0.10a |
0.54±0.04a |
|
T1 |
4.43±0.16b |
2.47±0.08b |
1.96±0.24ab |
1.31±0.20b |
|
T2 |
5.79±0.29c |
3.92±0.19c |
1.87±0.01ab |
2.09±0.08c |
|
T3 |
6.32±0.25c |
3.97±0.02c |
2.35±0.27b |
1.73±0.21bc |
|
p-value |
<0.001 |
<0.001 |
0.157 |
0.001 |
Table 3 Serum protein profiles of Cyprinus carpio fed different doses of LATP-Ca experimental diets
|
Treatments |
GLU |
CHOL |
TRIG |
CREAT |
B-Urea |
|
Control |
1.15±0.04d |
55.35±0.18d |
6.75±0.84d |
1.07±0.03d |
13.75±0.33d |
|
T1 |
1.40±0.07c |
53.35±0.11c |
6.55±0.18c |
0.90±0.03c |
13.45±0.47c |
|
T2 |
0.81±0.01a |
41.54±0.22a |
4.70±0.07a |
0.88±0.01a |
12.85±0.04a |
|
T3 |
0.94±0.02b |
48.45±0.18b |
5.40±0.22b |
0.95±0.03b |
13.40±0.22b |
|
p-value |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
Table 4 Serum metabolic profiles of Cyprinus carpio fed different doses of LATP-Ca experimental diets
|
Treatments |
SGOT(U/L) |
SGPT(U/L) |
ALP(U/L) |
|
Control |
575.34±36.12b |
145.10±4.21d |
68.05±0.77a |
|
T1 |
508.70±18086b |
99.85±1.41c |
178.40±3.34b |
|
T2 |
352.68±8.45a |
79.65±2.04b |
233.75±3.26c |
|
T3 |
338.48±3.99a |
26.10±2.07a |
279.90±6.69d |
|
p-value |
<0.001 |
<0.001 |
<0.001 |
Table 5 Serum protein profiles of Cyprinus carpio fed different doses of LATP-Ca experimental diets
Haemato-morphological performance
Haemoglobin, RBC, Hct content, MCV, MCH, and MCHC values are given in Table 6. The dietary ascorbic acid supplementation had a significant effect on the Hb, RBC, and Hct content, with the highest value recorded in T1 and T2 groups, respectively. Although statistically similar (p>0.05) value was observed between the other groups and control, but their values were found to be higher. A significantly higher (p<0.05) WBC count was recorded in fish fed 2000 mg/kg A.A. (T3) compared with the control. The MCV, MCH, and MCHC values did not differ (p>0.05) among the experimental groups.
|
Treatments |
HB(g/dl) |
RBC(106 cells/mm3) |
WBC(105 cells/mm3) |
HCT(%) |
MCV(fL) |
MCH(pg) |
MCHC(g/dl) |
|
Control |
3.95±0.37a |
0.32±0.32a |
13.36±1.31a |
30±0.57a |
948.98±111.72 |
122.15±0.28 |
13.22 1.50 |
|
T1 |
6.30±0.51b |
0.38±0.03ab |
28.61±1.54b |
40.50 0.86ab |
1053.36±74.71 |
166.99±28.92 |
15.62 1.61 |
|
T2 |
5.20±0.80ab |
0.52±0.07ab |
53.10±4077c |
46± 5.19b |
943.83±246.85 |
108.02±32.43 |
11.19 0.50 |
|
T3 |
5025±0.66ab |
0.48±0.07ab |
65.55±1.19d |
43±5.77ab |
962.55±271.66 |
108.50±2072 |
13.11 3.39 |
|
p-value |
0.138 |
0.133 |
<0.001 |
0.086 |
0.975 |
0.257 |
0.532 |
Table 6 Complete haemogram profile of common carp fed varied doses of LATP-Ca experimental diets
Considering, histo-morphology of the blood of Common carp, erythrocytes, thrombocytes, lymphocytes, monocytes, and neutrophilic granulocytes were identified based on nuclear morphology and cytoplasmic characteristics (Figure 5 – Figure 21). Erythrocyte assessment presented that, as the dose of vitamin C in the diet increased, a significant increase in the size of elongated oval-shaped erythrocytes were found (Figure 5). The size of RBC in control diet-fed carp ranged from 6.25 (Plate 339/Figure 5) to 8.84 μm (Plate 340/Figure 6), whereas the size in D1, D2, and D3 diet-fed groups ranged from 7.38 (Plate 341/ Figure 7) to 10.47 (Plate 342/ Figure 8), 7.69 (Plate 343/ Figure 9) to 11.25 (Plate 344/ Figure 10) and 9.56 (Plate 345/ Figure 11) to 11.40 μm (Plate 346/ Figure 12) in diameter, respectively.
However, fused erythrocytes were also observed in the blood smears of treated fish. In D1 (Plate 347/ Figure 13) diet-fed carp the size of fused erythrocytes ranged from 15.96 to 24.71 μm in length whereas the size in D2 (Plate 348/ Figure 14) and D3 (Plate 349/ Figure 15) diet-fed carps ranged from 23.22 to 25.37 and 23.26 to 24.71 μm, respectively. Erythrocytes had a central, rounded or oval nucleus and light, yellow-orange cytoplasm. Thrombocytes were smaller than RBCs, and the N: C ratio was higher than that of RBCs. Thrombocytes were oval and had a central nucleus with a rim of light gray cytoplasm (Plate 350). Thrombocytes of 6.7, 7.3, and 8.2 μm were recorded in D1 (Plate 350/ Figure 16), D2 (Plate 351/ Figure 17), and D3 (Plate 352/ Figure 18) diet-fed carps, respectively.
Lymphocytes of control diet-fed carp were 33 in number, and their size ranged from 7.31 to 8.6 μm (Plate 353/ Figure 19); the size of lymphocytes in D1, D2 and D3 diet-fed carps ranged from 6.8 to 8.6 (Plate 350/ Figure 16), 7.3 to 9.1 (Plate 351/ Figure 17) and 8.4 to 10.6 μm (Plate 354/ Figure 20), respectively. The numbers were 16, 3, and 8 in D1, D2, and D3 diets fed carp. Neutrophilic granulocytes were rounded and nucleated, 10.9 μm in diameter in D2 (Plate 351/ Figure 17) diet-fed carp, while the size of neutrophilic granulocyte in D3 (Plate 355/ Figure 21) diet-fed carp was 12.3 μm. The nucleus contained moderately condensed chromatin. Neutrophilic granulocytes had abundant, light eosinophilic cytoplasm with numerous fine cytoplasmic granules (Plate 355/ Figure 21).
L‐ascorbic acid (A.A.) is required for all vertebrate animals. It shows a vital role in the growth, collagen formation, iron metabolism, and hematology, reproduction, response to stressors, wound healing, and immune response.33 Fish cannot synthesize vitamin C or L‐ascorbic acid from D‐glucose due to the lack of L‐gulonolactone oxidase that is responsible for the synthesis of vitamin C de novo; therefore, an exogenous source of vitamin C is required in fish diet.34 Vitamin C has been one of the essential nutrients related to fish immunity.35 The quantitative requirement for several fish species studied shows far ranges between 25-200 mg A.A. kg-1 diets, depending on the age, size, species, stages of development, and culture conditions.36 However, due to the multiple roles of ascorbic acid in several metabolic processes, tissue formation, and immune responses, increased through the dietary allowance of common carp with A.A. 10 times more than the requirement for growth, and observed a significant improvement in growth and nutrient utilization indices.37 In the present study, the group of fish fed between 1g to 2g vitamin C (LATP-Ca) kg-1 performed better in percent weight gain, SGR, and FCR compared to the other fed groups. These answers are in agreement with several other studies for different fish species, such as olive flounder38 and cobia.39 An increase in growth has been reported to be a function of the nutritional quality of a diet and often considered as a positive index in nutritional requirement studies.40 Labh et al.41 reported that Cyprinus carpio fed diets containing a high level (800-2000mg A.A. kg-1) of vitamin C performed better in terms of growth and nutritional indices compared to the fish fed less or no ascorbic acid.
Similarly, Tewary and Patra et al.42 stated that L. rohita fed ascorbic acid supplemented diet showed higher SGR and lowered FCR value up to 1000 mg A.A./kg compared with the control group. The results indicated that dietary vitamin C could improve growth performance, and it is essential for normal physiological function. Hence, the increased growth observed in the present study can be attributed to the role A.A. in the process of tissue growth and repair and nutrient utilization, since ascorbic acid plays an essential physiological function in some aspect of protein metabolism and collagen formation.43
The absorption of vitamin C in various tissues is Figure 21 Morphology of erythrocyes (T3 diet fed carp) related to the dietary intake of the vitamin. Moreover, some tissues, such as the liver and head-kidney and leukocytes, accumulate in high concentrations. In these tissues, A.A. levels seem to be retained longer in the case of dietary vitamin C depletion compared to storage organs such as muscles.44 In the present study, increased dietary levels of ascorbic acid resulted in a significantly elevated level of A.A. in the tissue. However, a sharp decline in A.A. was observed above 1500 mg/kg supplementation level in all the tissues, thus indicating that the organs are saturated with ascorbic acid. This conforms with the previous report that excessive dietary A.A. cannot accumulate in the tissue once saturated,45 and requirements for tissue saturation are much higher than that for healthy fish growth for the prevention of deficiency symptoms (Lim and Lovell 1978). Similar reports were given in Lateolabrax japonicus;46 Epinephelus malabaricus;47 Oreochromis karongae;48 and Rachycentron canadum.49 A second-order polynomial was used to determine the exact maximal tissue level due to a decline in A.A. concentration above 1500 mg/kg tissue level. It was observed that the maximal storage level for C. carpio was 1355 mg/kg, 1532 mg/kg, and 1216.1 mg/kg for liver, muscle, and kidney, respectively. These levels will ensure adequate protection of the organ against oxidative insult and help in the regeneration of vitamin E after oxidation.
Several authors have also reported that the level of vitamin C required for maximum tissue storage is always higher than the requirement for maximum growth50–52 found a correlation between the dietary and liver A.A. concentrations in fish. Liver ascorbic acid content is usually considered as an indicator of the vitamin C status.53 In the red drum, liver storage of ascorbic acid in response to graded levels of dietary vitamin C was not as readily influenced, as seen in other species such as channel catfish.54 However, levels of total ascorbate in livers of red drum fed diets with supplemental vitamin C were generally similar to those reported for some other fish species.55–57
Serum total protein content plays a prominent role in determining the nutritional status and welfare of an animal, including fish. These circulating mobile proteins can further be grouped into albumin and globulin, whose role in the innate defense mechanism of fish has been reported.58,59 In the present study, total protein and albumin content were found to increase with the increasing addition of A.A., which is similar to the report of Rathore et al60 in tilapia. Except with fish fed 2000 mg/kg A.A., no differences were found in the globulin content of fish fed T1, T2, and control diet, wherein a significant increase was observed in the A/G ratio value. The higher A/G ratio recorded in this study is at variance to those reported by Labh et al.61 who recorded a higher A/G ratio in control (without A.A.). Although globulin data was not presented, we think that the reason for the disparity may be due to the low concentration of globulin in their A.A. supplemented groups compared with the control. Nevertheless, in the current study, the significant reduction in total protein, albumin and A/G ratio observed in the control group points in the direction of nutritional deficiencies and or depression of the immune function, since decreased albumin level is often associated with dietary deficiency and used as an index of liver damage.62 It should also be noted that protein plays a significant role in tissue repair, making its concentration low in the serum, as seen in the control fed group.63
Blood glucose, cholesterol, triglycerides, creatinine, and area act as primary and secondary responses to stress and have been used as indicators to determine the duration and severity of stress.64 In the present study, the inverse relationship observed among the treated and control diet-fed groups. Plasma glucose levels in fishes increase during stress, probably because of catecholamine action on stored glycogen in the liver and other tissues.65 Vitamin C acts as a regulator of catabolism of cholesterol to bile acid and has been demonstrated to be an essential factor in lipid regulation.66 The low creatinine values may indicate a disruption in the cyclization of creatine, which forms more than half of the urinary nitrogen of most fish.67 Urea level was significantly (P<0.05) higher in the control group compared to treated groups. Plasma urea has been used to compare the value determined by growth study on rainbow trout68 and European sea bass.69 Cardinaletti et al.70 indicated that postprandial serum urea concentrations in the rainbow trout could be as sensitive as growth in determining the arginine requirement of the fish.
The presence of some metabolic enzymes such as AST and ALT in the serum or plasma is often indicative of hepatic damage.71 The lower AST and ALT activities recorded in the group fed mega dose (1000-200 mg/kg) of A.A. might be due to ascorbic acid's role in the protection of tissues from oxidative damage.72 This damage usually occurs when ROS is overproduced, thereby inflicting damages on vital organs. However, ascorbic acid is a potent water-soluble antioxidant that helps counteract the effects of this ROS, possibly be the reason for the lower activities of these enzymes in the serum of the fish fed A.A. supplemented diets. According to Liu et al.73, feeding fish with diets low or devoid of vitamin C may result in reduced antioxidant capacity and more susceptibility to inflammation as a result of tissue damage. Higher serum ALP activity was recorded in the groups fed based diets, and this may be linked to the role of A.A. in stimulating alkaline phosphatase activity, a marker for osteoblast function.74 Also, since ALP is a hydrolase enzyme that catalyzes the breakdown of phosphate-containing compounds in the body,75 so the increased activity of this enzyme in the present study gave an insight into the utilization of L-ascorbate-2-triphosphate-Calcium by the fish and its positive effect on the growth performance. The result of the present findings is in accord with the previous study of juvenile cobia Rachycentron canadum76 and tilapia Oreochromis niloticus77 fed A.A. supplemented diets.
Hematological indices are often employed to evaluate the physiological and health status of fish in response to different diets or environmental variables,78 and any reduction in these parameters is often related to their health conditions.79 The present study showed that A.A. supplementation had a positive influence on the Hb, RBC, WBC, and hematocrit value of the treatment fish compared to the control. The reason for the reduction in the value of Hb, RBC, and Hct in the control may be attributed to the nutritional deficiency often caused by the reduction in iron absorption, an essential mineral for blood formation. It is a well-documented fact that ascorbic acid deficiency could result in anemia in fish due to its role in the absorption of iron and, consequently, in the synthesis of hemoglobin.80 Cai et al.81 reported that juvenile hybrid grouper (Epinephelus fuscoguttatus ♀× Epinephelus lanceolatus ♂) fed diets supplemented with A.A. performed better than the control fish in terms of hematological indices.
A similar report was made by Zhou et al.82 in juvenile cobia Rachycentron canadum fed vitamin C supplemented diets. The lower RBC and Hb values observed in the control group could be said to be a sign of anemia, which may not be unconnected with the vitamin C deficiency as seen in most studies.83 White blood cell plays a significant role in the immune function of fish, and elevated level of WBC observed in the group fed 2000 mg A.A./kg diets indicated that adequately high level of A.A. could confer immuno-competency on C. carpio before any incidence of diseases and or stress condition. Tewary and Patra84 made a similar observation in L. rohita fed A.A. up to 1000 mg/kg, but a decreased WBC value was observed when the inclusion level increased to1500 mg/kg. The reason for the variation compared to the present study might be due to the form of ascorbic acid used (L-ascorbate-2-triphosphate-Ca) and species differences in the utilization of different forms of ascorbic acid.
Histomorphology of blood provides comprehensive diagnostic, prognostic, and therapeutic information on a broad range of hematology and disorders of peripheral blood. Erythrocyte assessment indicates better performance in carp fed with vitamin C treated group compared to the control diet-fed group. In addition to erythrocytes, white blood cells (WBC) are good indicators of physiological stress in fish, even though there are very few studies regarding these parameters, particularly on tropical fish.85 Leukocytes involved in nonspecific defense mechanisms are mainly monocytes/macrophages and granular leukocytes, such as neutrophils. Neutrophils are vital effector cells in nonspecific immunity because they migrate into the infection site, where they recognize, ingest, and destroy many pathogens.86 Monocytes, macrophages, and lymphocytes are involved in the specific immune response leading to the production of antibodies. In summary, the supplementation of ascorbic acid at higher doses showed a positive effect on the growth immuno-biochemical and blood morphological indices in C. carpio. The tissue concentration of AA was also significantly influenced by the feeding of a high ascorbic acid level. Therefore, it is suggested that the inclusion of a higher dose of AA should not be more than 1500 mg/kg in the diets of common carp for maximal tissue storage, and to confer immuno-competency on the fish before any incidence of diseases and or stress condition.
Under the Fellowship (102/TU-IOST-AMRITCAMPUS/2005-2007), this work was supported by the Tribhuvan University, Nepal.
None.
The author declares that there is no conflicts of interest.

©2024 Labh. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.