Advances in
eISSN: 2378-3168


Mini Review Volume 10 Issue 5
1Department of Population Health, Center for Healthful Behavior Change, USA
2Somnogen Canada Inc., College Street, Canada
3Division of Endocrinology, Department of Medicine, USA
Correspondence: Alyson K Myers, North Shore University Hospital, Manhasset, NY, USA
Received: September 02, 0020 | Published: October 29, 2020
Citation: Jehan S, Zizi F, Pandi-Perumal SR, et al. Energy imbalance: obesity, associated comorbidities, prevention, management and public health implications. Adv Obes Weight Manag Control. 2020;10(5):146-161. DOI: 10.15406/aowmc.2020.10.00321
The prevalence of obesity has been continually increasing, as have its associated comorbidities and health care costs. Effective management of obesity and early intervention measures are necessary to overcome this global issue. The responsibility for preventing and managing this global epidemic does not lie solely on an individual, but also on the entire health care system. Policy makers—nationally and globally—must play their roles to solve the issue. In this review article, we examine methods of controlling and managing obesity through interventions, such as a low caloric diet, physical exercise, pharmacological guidance, and bariatric surgical procedures. While health care professionals should educate patients about all available treatment options for severe obesity, bariatric surgical procedures have increased in popularity and are considered very beneficial with outcomes fruitful in managing severe obesity.
Keywords: bariatric surgery, comorbidities, diet, obesity, management
BMI, body mass index; CVD, cardiovascular disease; GBP, gastric bypass; HDL, high-density lipoprotein; HTN, hypertension; IDF, the International diabetes federation; MetS, metabolic syndrome; SA, obstructive sleep apnea; QOL, quality of life; SES, socioeconomic statuts; T2DM, Type 2 diabetes mellitus
Multiple socioeconomic, cultural, hormonal, genetic factors and energy imbalance play important roles in the etiology of obesity.1–7 Energy balance involves maintaining equal energy intake and consumption (i.e. physical activity). It should be maintained through a balanced, healthy diet and lifestyle. When this balance gets disturbed, this creates a negative energy balance, meaning more energy intake and less consumption leading to weight gain. To prevent weight gain to the point of obesity and manage obesity’s comorbidities, a low-energy diet and more physical activity should be encouraged.8–11
A high caloric diet and a lack of exercise play an important role in causing patients to be overweight or obese and who suffer from related chronic comorbid conditions, such as type 2 diabetes (T2DM), dyslipidemia, and hypertension (HTN), obstructive sleep apnea (OSA), or non-alcoholic fatty liver disease (NAFLD).12 Additionally, there is a higher mortality rate noted in overweight or obese patients compared to normal weight individuals.13 Patients and healthcare workers should take preventive measures to control obesity and its related complications. Treatments can be lifestyle modifications, pharmacotherapy or bariatric surgery. It is noted that there was a greater reduction of weight loss and associated comorbidities such as both T2DM and HTN for patients who underwent gastric bypass (GBP) surgery for their morbid obesity.14,15
Prevalence of obesity Worldwide
In America, the obesity prevalence from 1960-2004 increased from 13% to 32%, and it has continued to increase at an alarming rate.16 This is no different in the United States where according to the Centers for Disease Control the prevalence of obesity in 2017-2018 is as high at 42% (https://www.cdc.gov/nchs/products/databriefs/db360.htm) (Figures 1&2).
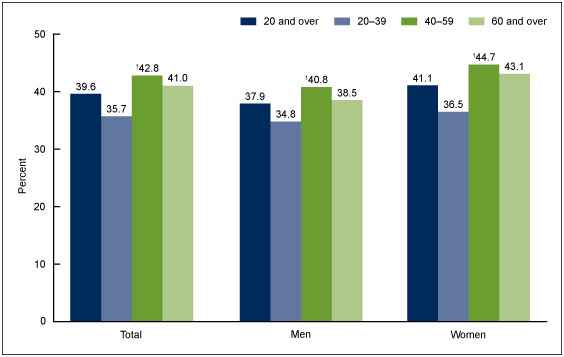
Figure 1 Prevalence of obesity among adults aged 20 and over, by sex and age: United States, 2015–2016.
SOURCE: NCHS, National Health and Nutrition Examination Survey, 2015–2016.
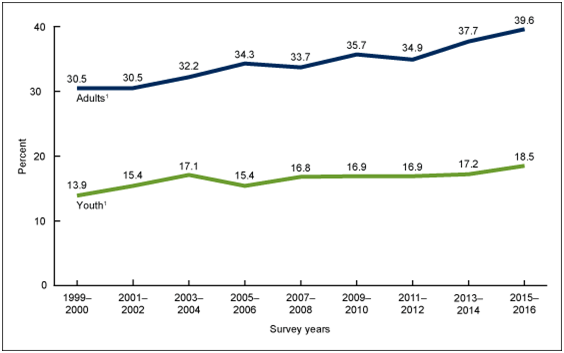
Figure 2 Trends in obesity prevalence among adults aged 20 and over (age adjusted) and youth aged 2–19 years: United States, 1999–2000 through 2015–2016.
Source: NCHS, National Health and Nutrition Examination Survey, 1999–2016.
Internationally, researchers used the Global Burden of Disease found that 603.7 million adults and 107.7 million children were obese in 2015. It is estimated that between 1980 and 2015, obesity doubled in more than 70 countries. Four million deaths were reported globally due to high BMI, and more than two-thirds of those deaths occurred due to cardiovascular disease (CVD) from 1990-2015 in 195 countries globally.17 In 2005 the world’s adult obese population was 9.8% (9.6-10.0%). The estimated obese adult individuals were 396 million (388-405million). If the secular trends will be continued then it is estimated that by 2030, the total number of obese adult individuals were projected to total 1.12 billion.18 It has also been projected that by 2030, 65 million Americans and 11 million British persons will be obese and have associated comorbidities such as cancers, T2DM, and CVD. The estimated combined medical costs for the treatment of obesity and its related comorbidities in the US will be increased $48-66 billion per year and in the UK £1.9-2 by 2030.19
Etiological factors of obesity
The etiology of obesity can be characterized as multifactorial and includes the effects of hormones, genetics and environment.20
Obesity and hormonal disturbance
Hormones such as ghrelin and leptin are involved in the regulation of weight, as well as energy consumption and appetite. Ghrelin, which is secreted from the enteroendocrine cells of the gastrointestinal tract, is usually called a “hunger hormone” because it stimulates the hunger center in the hypothalamus. In contrast, leptin, which is secreted from the adipose tissues, is called the “satiety hormone” because it suppresses food intake and sends signals to the brain satiety center. In normal individuals, these hormones regulate the hunger-satiety mechanism, which in turn regulates the normal weight of an individual. Disruption of these mechanisms leads to hyperphagia, resulting in weight gain and severe obesity (Figure 3) .21,22
Congenital leptin deficiency shows disturbance in the circadian rhythm of hunger and satiety, which leads to unsuppressed hyperphagia and obesity.23,24 In those with hyperphagia, an increased level of leptin is found within their plasma; however the brain cells cannot recognize these high levels and instead perceive a low level of afferent signals of leptin while the ghrelin continues to secret, thus resulting in an increased demand for food intake and, ultimately, severe obesity (Figure 4).25
Obesity and genetics
There are three main classes of transmission of obesity through genes: monogenic obesity, polygenic obesity, and syndromic obesity.26
Monogenic obesity
There are primarily five genes involved in monogenic transmitted obesity. Leptin, the leptin receptor, melanocortin-4 receptor (MC4-R), pro-opiomelanocortin (POMC), and proconvertase 1.27 MC4-R is found to be the most commonly inherited obesity gene among and has been found in 5-6 % of severely obese people. In the general population of the United Kingdom, this mutation was noted in 1/1,000 persons. Severely obese patients showed mutations in MC4-R and a gene deficiency on chromosome (18q21.32), which leads to hyperphagia, hyperinsulinemia, and obesity. Another example of the most commonly monogenic transferable gene is the leptin (LEP) gene mutation. In the LEP gene mutation, monogenic obesity is caused by the mutation in the single gene of LEP, in which the chromosomal mutation is (7q32.1). The leptin receptor gene mutation is on chromosome (1p31.2). The mode of transmission of these genes is autosomal recessive.6,24,26,29
Polygenic obesity
A polygene of obesity is due to a defective allele that is formed on the same chromosome because of a mutation of a gene that increases body weight in obese individuals. From the population perspective, one potential example could be the prevalence (0.02%) that was noted in a rare pathogenic loss-of-function of the MC4-R variant that introduces a premature stop codon—p. Tyr35Ter. This, in turn, is essentially inherited as a shared haplotype with a missense mutation, or p. Asp37Val.20
Syndromic obesity
Syndromic obesity could be due to a mutation in one gene, or a larger part of chromosome can be affected due to mutations in multiple genes. Furthermore, it could be associated with other phenotypic deformities, such as neurodevelopmental defects along with the severe onset of early obesity. The most common examples of syndromic obesity are Prader-Willi and Bardet-Biedl syndromes.26
Prader-willi syndrome
Prader-Willi syndrome (PWS) is the most common example of syndromic obesity in the world. It is a complex neurodevelopmental disorder in which the genetic defect is located on the 15q11-13 region of the paternal chromosome. It is characterized by severe infantile hypotonia, and poor suck and feeding difficulties. There are two stages of eating disorders in PWS; poor feeding in the first stage during early infancy can lead to anorexia and failure to thrive. Stage one can be followed by excessive hyperphagia and severe obesity, which is called stage two of early childhood. Hypogenitalism, hypogonadism, short stature, and cognitive impairment are also features of PWS.30–33
Bardet-biedl syndrome
Another common example of syndromic obesity is Bardet-Biedl syndrome (BBS). BBS is an autosomal recessive ciliopathy that is characterized by obesity, retinal dystrophy, polydactyly, cognitive impairment, renal abnormalities, and urogenital anomalies. Proteins that are present on basal body and cilia are encoded by BBS genes and the mutation results in ciliopathy rather than normal cilia biogenesis and functioning (Figure 4).34–36
Environmental factors
Obese children have a higher chance of becoming obese adults, as many factors can play a vital role in maintaining obesity from childhood to adulthood, such as environmental, behavioral, and genetic factors.37 The easy availability of high-caloric food—such as sugary drinks—at home and schools, and physical inactivity—such as watching TV for multiple hours—put children at greater risk of obesity.38 Food industries are globally producing more processed food, which is often cheaper than fresh fruits or vegetables, thus contributing to rising global obesity.39 Television ads of processed food and sugary drinks have been associated with childhood health and obesity.2 Studies have shown an increase in children’s consumption of fast food after watching television advertisements from Subway, Wendy’s, and McDonald’s.40 Similarly, children consumed more high-sugar breakfast cereals after watching advertisements on TV.41,42 Studies have shown a linear relationship between the increase in weight throughout the year in pre-school children who regularly consume fast food in an average week (P<0.01).42 Studies also show the presence of certain chemicals in the air due to pollutants from chemicals industries that can accumulate in human bodies and cause obesity. Such chemicals include: dichlorodiphenyldichloroethylene, dichlorodiphenyltrichloroethane, and polychlorinated biphenyls, etc. These pollutants are mainly lipophilic compounds and they can easily be stored in adipose tissues, resulting in a predisposition to obesity.18,43,44 Sleep disturbance, depression, mother’s lack of education, and childhood abuse also lend to gaining obesity. Eating disorders such as bulimia can also be the causative factor of obesity. To overcome obesity and its comorbidities, these mental and psychological issues should be addressed during childhood or at the earliest age of diagnosis.45–47
Socioeconomic status
Socioeconomic status (SES) plays an important role in obesity as higher SES is associated with higher BMI and cardiometabolic risk among adults.1 This applies to both underdeveloped as well as low to middle income countrie.48,49 A Mexican study showed that higher SES levels are directly related to increasing obesity prevalence due to the greater access to high calorie items (alcohol, soda, candy, chips) as compared to lower SES.50 Interestingly, the inverse was found to be true in underweight populations in India (Table 1).51
|
N men |
N women |
Age (years) |
SES |
BMI |
Waist circumference |
P value |
References |
|
987 |
819 |
21.5 |
higher |
≥25kg/m2 |
Women > 80 cm, |
Men = P< 0.05 |
171 |
|
Men > 85cm |
|||||||
|
1301 |
1530 |
≥ 25 |
higher |
≥30kg/m2or 25-29.9kg/m2 |
Women ≥ 80cm, men ≥ 94cm |
Overweight= (P < 0.08), |
1 |
|
Obese = (P< 0.001) |
|||||||
|
94 656 participants |
>19 |
higher |
≥25kg/m |
|
|
137 |
|
Table 1 SES and obesity prevalence
On the other hand, some studies conducted in developed countries show an inverse relationship between higher SES and obesity; a nationwide study conducted in Poland showed a higher SES and a lower BMI by adopting a better life style, such as by reducing smoking, increasing regular physical exercise, and implementing a healthier diet.52In women in developed countries, a higher SES has been associated with a lower BMI; whereas obese women tend to exhibit a lower SES.53–55 Education also plays an important role in a person’s lifestyle; a study showed higher education levels are associated with lower BMI in both sexes, but only women showed an inverse relationship with abdominal adiposity.56 Overall the prevalence of obesity from 1988-1994 and from 2007-2008 increased at all income levels, and at all levels of education (Figure 5).57
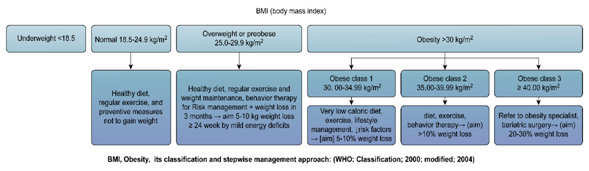
Figure 5 BMI< Obesity its classification and stepwise management approach: (WHO: classification; 2000; modified ; 2004.
Obesity, morbidity and mortality
Severe obesity, especially classes 2 and 3, are associated with higher morbidity and mortality.58,59 Obesity is associated with a lot of comorbidities including: T2DM, cancer, CVD, asthma, osteoarthritis, chronic back pain, OSA, NAFLD and gallbladder diseases (Figure 6).60,61A cohort analysis was assessed on employees’ overall health, obesity, and associated comorbidities. The results showed that there was decreased work production, more health problems, and an increased annual health cost budget among obese employees (Table 2).62
|
BMI (kg/m2) |
< 27 |
27 ≤30 |
≥30 |
P-value |
Reference |
|
Number of Employees |
39,696 |
14,281 |
18,801 |
||
|
Per-employee adjusted total annual costs |
$4,258 |
$4,873 |
$6,313 |
(P < 0.001) |
62 |
Table 2 Obesity and Associated Comorbidities: Annual Cost of Employees
The English Longitudinal Study of Ageing was a population-based cohort study was conducted to understand the effects of morbidity and mortality in individuals age ≥ 65 years who were overweight or obese in comparison to those who were of normal weight, was conducted among 3,709 with the follow-up period of five years. Patients with a BMI 30.0-34.9 had a greater difficulty in activities of daily living (ADLs) RR 1.99 (95% CI = 1.42-2.78), functional impairment of RR 1.51 (95% CI = 1.05-2.16), and mortality RR 0.99 (95% CI = 0.60-1.61).63 An Austrian cross-sectional study examining obesity and mortality demonstrated a U-shaped association was found in BMI and all-cause mortality. The highest association was found with the BMI ≥ 35, with a hazard ratio (HR) in women = 1.60 (95% CI, 1.42-1.81), and in men = 2.13 (95% CI, 1.82-2.48).64 Another study in postmenopausal women shows that severe obesity is highly associated with a higher risk of mortality. Normal BMI, hazard ratios (HRs, 95% confidence interval), compared to severe obesity mortality were in Hispanic women; 2.59 (1.55-4.31), in white; 1.97 (1.77-2.20), and in African American; 1.55 (1.20-2.00).65 An increase in BMI at an early age is also a predictor of obesity-related comorbidities and early death in later in life. So, the early preventive measures and management approaches are highly recommended to overcome this growing epidemic globally (Tables 3&4).66
|
Age (Years) |
Gender |
Smoking history |
Overweight/obese |
↓Life expectancy (Years) |
Counterpart |
Reference |
|
40 |
M |
Nonsmokers |
Overweight |
3.1 |
Normal weight smokers |
66 |
|
40 |
F |
Nonsmokers |
Overweight |
3.3 |
||
|
40 |
M |
Nonsmokers |
Obese |
5.8 |
||
|
40 |
F |
Nonsmokers |
Obese |
7.1 |
||
|
40 |
M |
Smokers |
Obese |
6.7 |
||
|
40 |
F |
Smokers |
Obese |
7.2 |
|
|
Table 3 Reduction in life expectancy, overweight/obese: The Framingham Heart Study (1948-1990)
|
Age |
Gender |
Smoking history |
obese |
↓Life expectancy (Years) |
Counterpart |
Reference |
|
40 |
M |
smokers |
obese |
13.7 |
Normal weight nonsmokers |
66 |
|
40 |
F |
smokers |
obese |
13.3 |
|
|
Table 4 Reduction in life expectancy, obese: The Framingham Heart Study (1948-1990)
Central adiposity is a further predictor for disease activity and mortality in obese patients. In a study of adults aged 20 to 83 years, there was a linear association of waist circumference in men with all-cause mortality (HR, 1.52 for waist circumferences of ≥ 110 vs< 90cm; 95% CI, 1.45-1.59; HR, 1.07 per 5-cm increment in waist circumference; 95% CI, 1.06-1.08). In women the association was seen at a smaller measurement (HR, 1.80 for waist circumferences of ≥ 95 vs< 70cm; 95% CI, 1.70-1.89; HR, 1.09 per 5-cm increment in waist circumference; 95% CI, 1.08-1.09). Additionally, there was a 3-year decrease in the life expectancy of men with higher waist circumference compared to lower waist circumference, and a 5-year decrease for women (BMI=20-50kg/m2). Waist circumference so should be assessed along with BMI as some normal or underweight persons may have central adiposity despite having a BMI less than 25.34 A study among 26,144 women and 14,585 men called the “Swedish National March” was conducted and followed up for 10 years to see all cause-mortality. Results showed that women with waist circumference ≥ 88cm had double the risk of mortality compared to normal weight, physically active women with a waist circumference of < 80cm. Men with sedentary lifestyles and a BMI ≥ 30 had an increased rate of mortality when compared to physically active and normal BMI counterparts.67 Extreme obesity will increase the all-cause mortality rate, even among young premenopausal women; In a cohort of women from Norway and Sweden aged 30-50, followed longitudinally from 1991 to 2000, the mortality risk became doubled for women with BMI=30 (HR=2.2, (95% CI: 1.7-3.0) when compared to women with normal BMI (18.5-24.9).68
Preventive measures
Prevention of obesity is the responsibility of individuals, friends, family and health care workers. Individuals are responsible for eating healthy and staying active. However, this is a cycle which not only involves an individual’s responsibility but also emphasizes the need to involve policymakers and government in making rules and laws and implementing them. Food industries should play their essential part in providing healthy food to the community. The industries should provide healthy and affordable food. Policymakers who are involved in making the rules at the government level should be involved in how the policies will be enacted for those of all SES or demographic.69–72
Governments frame the entire food system by monitoring the food from production, processing, distribution, marketing, retail, and catering until the delivery to the consumer. These policies also aim to increase and promote physical activity, creating school education programs for children to eat healthy and become involved in sports and other extracurricular physical activities. At workplaces, the guidelines and other information regarding healthy eating and involvement in physical activities are being encouraged and implemented.73, 74 The government should also provide safe places for public walking, running, and cycling. Some have suggested that taxes should be increased on alcoholic and sugary beverages, tobacco, and fast food. Public health should be the main concern of national and international policies, ensuring that healthy food should be accessible to prevent obesity globally.75–77 Also support of programs should involving school physical activities is required to overcome the growing epidemic of obesity.78
Treatment implications
To prevent and manage weight gain/obesity, the etiological factors of weight gain/obesity should be known first and addressed accordingly.72 Lifestyle modification, diet, exercise, medications, or bariatric surgery should be considered to lose weight.53,61,79
Lifestyle modifications
Sedentary lifestyle, lack of physical exercise along with high caloric diet are the predisposing factors of weight gain and obesity.80 Lifestyle modification involves teaching patients about diet, physical activity, and behavioral modifications; this should be the first-line therapy in obesity treatment.81 A Chinese prospective cohort study to examine the effects of lifestyle modifications on all-cause mortality. The results showed that carrying out healthy lifestyle factors (daily exercise, high fruit and vegetable intake, never smoking or exposure to smoking), as well as have a lower waist: normal BMI was associated with lower mortality.82 These lifestyle modifications play an important role in disease prevention and progression and decrease mortality.79
Diet control
A meta-analysis was performed to see the effects of weight loss by using lifestyle modifications on obese diabetic patients. The restriction of energy intake and low caloric diet greatly helped in weight management and improved glycemic control of those with T2DM. A very low-calorie diet reduced fasting blood sugar levels by 50% in two weeks. After 12 weeks significant decreases were noticed in body weight (9.6%), fasting blood sugar (25.7%), systolic BP (8.1%), diastolic BP (8.6%), serum cholesterol (9.2%), and serum triglycerides (26.7%).83 Eliminating consumption of high-glucose drinks can also help. Drinking 1-2 servings of sugar-sweetened beverages daily raises the risk of MetS or T2DM greater than those who have one drink a month or none at all [relative risk (RR), 1.26 (95% CI 1.12-1.41)]. The intake of these sugary drinks should be reduced to prevent obesity, and other components of the MetS.84 The Atherosclerosis Risk in Communities (ARIC) study was conducted among 9514 participants to see the connection between dietary intake and the occurrence of incidental MetS. Participants aged 45-65 years developed incidental MetS if their diet consisted of meat (p trend< 0.001), and fried foods (p trend = 0.02). Participants who consumed fruits, vegetables, whole grains, refined grains, nuts, and coffee, they didn’t show any association with incident MetS.85 Late night eating is also associated with weight gain. One study showed that short sleepers (fewer than 7 hours) had an improvement in metabolic indices and greater weight loss when they increased their sleep to more than 7 hours.86 Short sleepers (6 hours or fewer) who go to sleep late tend to eat later at night, increasing their daily intake of calories and becoming more prone to gain weight.87
Physical activity
Normal BMI and regular physical activity are the good predictors of good health and significant lower health risk of CVD, cancers and all other causes of mortality compared to people who have a sedentary lifestyle with higher BMI ≥ 30kg/m2.88 Exercise plays an essential and fundamental role in preventing obesity and maintaining an individual’s normal weight. Regular exercise and adherence to exercise programs show a significant decrease in an individual’s body fat and weight.89 Even if exercise doesn’t directly cause a person to lose weight, it still improves blood pressure, serum lipids level, glycemic control, and risk of CVD.89,90 Data collected from 3476 participants in 43 randomized control trials was demonstrated that even exercising alone without diet control had a significant decrease in fasting blood sugar; magnitude of weight loss (WMD -0.2mmol/L; 95% CI -0.3 to -0.1). Higher impact exercise had a greater impact on fasting blood sugar (WMD -0.3mmol/L; 95% CI -0.5 to -0.2). There were also reductions noted in triglycerides (WMD -0.2mmol/L; 95% CI -0.3 to -0.1) and diastolic blood pressure (WMD -2 mmHg; 95% CI -4 to -1).91
Exercise and diet control: enhancing further weight loss and improvements in comorbidities
Regular physical exercise along with dietary restrictions plays an important role in losing weight.91,92 Also demonstrated improvements in apnea-hypopnea index, and glycemic control when compared to those who had no interventions. Study also showed that the WMD was increased when combined with diet control; (WMD- 1.1 KG, 95% CI -1.5 to -0.6). When exercise intensity was increased the WMD was also increased, (WMD -1.5 kg; 95% CI -2.3 to -0.7). A randomized controlled trial study conducted in Netherlands found that caloric restriction and exercise led to more weight loss than caloric restriction alone or placebo in postmenopausal women. In addition to greater weight loss, there was also a decrease in intra-abdominal and subcutaneous fat.93
In older (age ≥ 60) overweight (BMI ≥ 28 kg/m2) individuals, exercise and diet control can improve osteoarthritis of the knee and self-reported function and pain (Messier et al, 2004). Study showed that in overweight/obese individual diet-only treatment did not show any improvements in pain [standardized mean differences (SMD) -0.13; 95% confidence interval, CI: -0.37, 0.10; I2 = 49%)] but diet+exercise showed improvements in pain (SMD -0.37; 95%CI: -0.69, -0.04; I2 = 54%). Physical function improved moderately with diet-only (SMD -0.30; 95%CI: -0.52, -0.08; I2 = 47%) and diet+exercise (SMD -0.32; 95%CI: -0.56, -0.08; I2 = 24%).94
Behavioral therapy
Behavioral and cognitive behavioral therapy (CBT) could be helpful in programs designed for the treatment of weight loss. In these treatments, people are encouraged to change their eating habits and adhere to a regular exercise regime.95,96 It is established that, among psychological and behavioral treatments, CBT is the best approach for weight management programs. CBT is the most effective treatment for eating disorders—especially binge eating.97 In one weight-loss study, a group was enrolled in weight-loss behavioral therapy in which their weight was monitored after the start of each month. Participants in the group who self-monitored their daily diet, exercised regularly, and weighed themselves on a regular basis showed a greater weight loss when compared to those who exhibited an in adherence to self-monitoring of diet, physical exercise, and weight measurement.98
Participants who participated in a behavioral therapy weight loss program lost 6.6 lb. in 12-18 months, a higher number compared to the contro.99 In a study of 430 patients, ages 49±9years, with BMI, 41±6kg/m2 and baseline waist circumference (WC) was 120±14cm, participants enrolled in an intensive behavioral weight management program for two years. At six months follow-up, BMI decreased by 6±3 kg/m2, waist circumference 14±9 cm, systolic blood pressure 8 mm Hg, glycated hemoglobin, (-1.2 vs-0.3%, p=0.006), total cholesterol, (-29 vs-12mg/dl, p=0.017), and low density lipoprotein cholesterol (LDL), (-19vs -4mg/dl, p=0.033). At 2 years follow up, BMI decreased by 5±4Kg/m2, and the WC by 11±11cm, so the decrease in BMI and WC greatly improve the MetS components as well.100
Pharmacological management
Pharmacological agents can be used to lose weight for long term management as adjuvant therapy along with lifestyle changes. In an intensive behavioral therapy (IBT) weight loss program, dietary self-monitoring alongside the use of a liraglutide (3.0mg/d) showed greater weight loss than only with dietary self-monitoring.101 Several anti-obesity drugs are currently approved for use including: Naltrexone-Bupropion (Contrave), Phenteramine-Topiramate (Qsymia), Liraglutide (Saxenda), and Orlistat (Alli 60mg capsules) or(Xenical 120mg capsules).102–106
Orlistat was approved in 1999 by the Food and Drug Administration (FDA) to treat obesity. This drug inactivates gastrointestinal lipases, thus causing the inhibition of dietary fat to be metabolized into its absorbable forms.107,108 A double-blind, prospective study was conducted for a 4-year time period in 3,305 patients with the BMI≥30kg/m2and a normal glucose level (79%) or impaired glucose tolerance (IGT) (21%).To treat obesity and to see the incidence of T2DM, orlistat, a pharmacological agent, was added with lifestyle modifications. After four years of treatment the results showed that for (lifestyle modifications+orlistat), the T2DM incidence was 6.2%, while for (life style modifications + placebo), the T2DM incidence was 9.0%, the mean weight loss with orlistat was (5.8 vs. 3.0 kg with placebo; P<0.001). Therefore, treating obesity with life-style modification along with a pharmacological agent (orlistat) showed better results and outcomes as it not only leads to a decrease in weight, but also the incidence of T2DM.109
The FDA approved phentermine/topiramate ER in July 2012 as an anti-obesity drug. A phen/tpmCR combination is associated with a greater weight loss when utilized along with life-style modifications than a single-drug therapy (orlistat) and is an FDA-approved pharmacological agent for long-term management of weight loss in obese people. A randomized controlled study in 3 groups of patients was conducted for 56 weeks to see the effects ofphen/tpmCR—low-dose [3.75/23 mg (n=241), high-dose [15/92 mg (n=512)], and placebo (n=514)—along with low calorie diet. The participants were between 18-70 years old with a BMI≥35kg/m2. Weight lost from baseline body with high-dose PHEN/TPM CR was 10.9%; with a low dose it was 5.1%; and with placebo it was 1.6% at 56W (P<0.0001), so the combination of these drugs at higher doses have more beneficial effects in the long-term management of obesity.110
Several drugs have been withdrawn from the market due to their increased and harmful side effects. Sibutramine was approved by the FDA in 1997 but was withdrawn in 2010 because of its increased risk for psychiatric disorders, myocardial infarction, and stroke.111 Fen-phen (fenfluramine/phentermine) was also withdrawn from the market because of its connection with valvular heart disease. It was withdrawn from the market on September 15, 1997.112 Locaserin (Belviq) was taken off the market in February 2020 due to an increased risk of cancer (https://www.fda.gov/drugs/drug-safety-and-availability//a>).
Bariatric surgery and its beneficial outcomes
Bariatric surgery is becoming a common treatment option for patients for further weight loss beyond lifestyle modifications.113–115 Bariatric surgery should only be performed when patients are fully informed about the details of the procedures, including the risks. Patients with BMI ≥ 35 kg/m2 who have severe comorbidities (i.e. T2DM, OSA) or with BMI is≥40kg/m2are eligible. Bariatric surgical procedures greatly help in reducing weight in severely obese patients where lifestyle modifications and other measures of weight loss fail to lose weight. There were drastic improvements noticed after these procedures not only in weight reduction but also in HTN, CVD, stroke, and they also showed reduced end-organ damage compared to the control group.116,117 After losing weight through bariatric surgery, OSA and metabolic derangements also greatly improved.118
Bariatric surgical procedures
Bariatric surgery helps patients to lose weight by restricting the amount of food the stomach can hold or by causing malabsorption of the nutrients of food, or a combination of both. These procedures also cause hormonal changes which affect and regulate the hunger mechanism. The main bariatric surgical procedures are gastric bypass (GBP) or the Roux-en-Y Gastric Bypass (RYGBP), laparoscopic adjustable gastric band (LAGB), sleeve gastrectomy (SG), and Biliopancreatic Diversion with Duodenal Switch (BPD/DS).119–124 Significant weight loss was noted after LRYGBP, LAGB, and LSG however, LRYGB procedures have a better outcome and greater reduction in BMI as compared to LAGB and LSG on a long-term basis.125
Gastric bypass (GBP) or the roux-en-Y gastric bypass (RYGBP) Procedures
It is noted that there was a greater reduction of weight loss and associated comorbidities such as both T2DM and HTN for patients who underwent GBP surgery.14
LRYGBP procedures
Lap RYGBP (LRYGB) procedures are considered the gold standard for weight loss and obesity-related comorbidities.115,124,126 LRYGB surgeries greatly improve weight loss and overall improve comorbidities in obese individuals. LRYGB surgery is more effective when BMI < 50 compared to when completed on a patient with a very high BMI.127–130
In LRYGBP procedures, the first step is to create a 15-30 ml pouch on the lesser gastric curvature using the stapling device, then an incision is made in the small intestine. The portion of the small intestine, which is called the duodenum, where most of the nutrients are absorbed, is bypassed along with the lower remaining portion of the stomach. The jejunum is pulled up and connected to the newly constructed small pouch of the stomach. The duodenum is then reconnected at the lower point to the jejunum (100-150 cm distal to the gastro-jejunal anastomosis). This entero- anastomosis restores the bowel continuity, which still adds gastric acid and digestive enzymes along with bile and pancreatic juices in the food for digestion. This bypass decreases hunger and increases satiety, which helps in dramatic weight loss. HTN, T2DM, and dyslipidemia were also significantly improved after LRYGBP.131–134
LRYGBPprocedures are minimally invasive procedures that are safe, feasible, and preferred over open RYGBP because of the short hospital stay and low perioperative morbidity. Additionally, weight loss after five years of LRYGBP is markedly improved; a study showed that, in a 5-year period, of the 1160 patients who had a LRYGBP, 240 had T2DM or impaired fasting glucose. Out of these 240, 191 patients showed up for a follow-up study, with a mean preoperative age of 48years. In these patients, the average post-surgical weight loss was from 308lbs. to 211 lbs., with a BMI decrease to 34kg/m2 from 50.1 kg/m2. In these patients, glycosylated hemoglobin concentration and fasting plasma glucose levels became normal (83%), or greatly improved (17%), and the complete resolution of T2DM occurred in the mildest form, which was controlled by the diet.135 Study data was similarly collected from 95 patients who underwent LRYGB with a starting BMI of 47+/-5kg/m2 and hyperlipidemia that was developed within 44+/-56M. After LRYGBP, 68 women with a mean age 43+/-10 years showed 66% loss of body weight, total cholesterol level ↓16%, TG ↓63%, LDL ↓31%, VLDL ↓74%, and HDL ↑39% in one year, indicating that the lipid profile was also greatly improved after LRYGB.136
Another review collected data of 219 patients who underwent and followed up for 26.4+/-12.8M. At their one-year follow-up, patient’s HGBA1c decreased from 7.6 to 6.1 and their fasting plasma glucose decreased from 152.8 to106.0 mg/dl. Total cholesterol changed from 180.9→172.0mg/dl, TG 208.0→117.4mg/dl, and HDL-C 48.7→58.7mg/dl. These data significantly indicate that patients showed significant improvements in their lipid profile and glucose metabolism after LRYGB.5
Laparoscopic adjustable gastric band (LAGB), or lap-band surgeries
Lap-Band surgeries are also beneficial in the reduction of severe obesity, as they not only cause weight reduction but can potentially decrease all associated comorbidities related with obesity. In LAGB procedures, the size of the stomach is reduced by placing an inflatable silicon band around the upper part of the stomach, which creates a small pouch resulting in the reduction of food intake. This limiting intake decreases hunger and produces satiety, which results in weight loss.137 Following their Lap-Band procedures, patients were greatly satisfied after a gross improvement in their quality of life (QOL).138 There was a significant improvement noticed in each patient’s overall physical and mental health. Significant improvements were noted in blood pressure, insulin sensitivity, and lipid levels. Pregnancy outcomes and fertility rates were also increased after a great reduction in weight loss through these bariatric surgical procedures.139,140 LAGB outcomes have proven to be good in regard to short-term weight loss, but long-term weight loss results are lacking, and reoperations may be required to maintain weight loss by this surgical procedure.141 One complication of LAGB is the slippage of the band which can cause it to become nonfunctioning, so reoperations are required to reposition the band. LAGB can be removed or converted to LRYGBP.142
Laparoscopic sleeve gastrectomy (LSG)
LSG is also one of the most common and widely used procedures worldwide. In this bariatric surgical procedure, a solid rubber tube is inserted into the stomach through the mouth. The stomach is divided into two parts through the use of a stapler, starting 4-6 cm from pylorus to give it a new shape similar to that of a banana. In this way, 75-80% of the stomach is removed through a small incision in the abdominal cavity. The removed part of the stomach contains the fundus of the stomach, which secrets the hunger hormone (ghrelin). Due to the lack of secretions from this hormone and the fact that the new size of the stomach has 20-25% capacity of the original stomach, a patient feels less hungry and achieves full satiety after eating a smaller amount of food, which ultimately leads to a significant weight loss.143–149
In a study with data collected from 20 articles, LSG was performed on 2713 adult patients with mean BMI 46.9kg/m2. Of the 1626 patients showed up for their follow-up. Among these patients the mean %EWL was at 5Y→58.4%, 6Y→59.5%, 7Y→56.6%, 8Y→56.4%, 11Y→62.5%. After 5Y of follow up, T2DM improved or resolved→ 77.8%, OSA→75.8%, HTN→68.0%, dyslipidemia→65.9%, degenerative joint disease→55.7%, and gastroesophageal reflux disease (GERD) →30.6%.150 Also, in obese renal transplant patients LSG greatly reduced the occurrence and severity of post-transplant complications.151
Biliopancreatic diversion with duodenal switch (BPD/DS)
BPD/DS are considered excellent bariatric surgical procedures for weight loss and for improving QOL.122Scopinaro et al.,152 study showed that these procedures normalized almost all components of MetS (HTN, FBS, and serum lipids levels), and patients were followed up for over ten years after their surgery.
Overall outcomes of bariatric surgical procedures
In a prospective study conducted in Sweden among 845 patients who underwent surgery and 845 in the control group, which were present in the conventional weight loss obesity treatment, the participant’s BMIs measured 41+/-4.6kg/m2. Patients who went for surgery lost on average 28+/-15kg, andthe control group only lost 0.5+/-8.9kg (P<0.0001). After a follow-up period of two years, the patients who were treated surgically showed better outcomes for T2DM, HTN, and dyslipidemia compared to those who were treated conventionally (Table 5).113
|
LRYGB |
|
LAGB |
|
LSG |
Reference |
|
%EWL |
%EWL |
%EWL |
%EWL |
%EWL |
125 |
|
≥5Y |
≥10Y |
≥5Y |
≥10Y |
≥5Y |
|
|
62.58 |
63.52 |
47.94 |
47.43 |
53.25 |
|
Table 5 A metanalysis: Excessive weight loss (EWL) in different Bariatric surgeries
The Swedish Obese Subjects (SOS) study in which patients were randomized to surgery (n=1210) for severe obesity versus a control group (n=1099). Obese patients who were treated surgically for their severe obesity lost 28kg of weight compared to the control group, in which there was not any significant weight loss. Surgically treated patients found a marked improvement in their OSA, T2DM, HTN, dyslipidemia, chest discomfort, and in dyspnea. Their physical activity time was also increased compared to the control group.153 Another SOS study was conducted from September 1st, 1987 to January 31st, 2001 to see the overall effects of bariatric surgical procedures on microvascular complications in obese patients according to their baseline glycemic level. In this study, patients ages 37 to 60Y withBMI≥34kg/m2 (men),≥38kg/m2(woman) and glycemic levels at baseline euglycemia→2838, prediabetes→591, screen detected T2DM→246 and established T2DM→357, were enrolled in a surgical group (n=2010) and control group (n=2037) that received only usual care. At median follow-up of 19 years, incidental microvascular disease was diagnosed in 374 patients in the control group and 224 patients in the bariatric surgery group (hazard ratio [HR] 0·56, 95% CI 0·48-0·66; p<0·0001). The subgroup showed unadjusted HRs; euglycemia (0·63, 0·48-0·81), prediabetes, (0·18, 95% CI 0·11-0·30), screening detected T2DM (0·39, 0·24-0·65), and established T2DM (0·54, 0·40-0·72). These results show that bariatric surgery reduces microvascular complications, especially in those patients who exhibit a prediabetic state at baseline.154
A large retrospective cohort study was conducted in obese adults from 1996-2009 with T2DM to see the outcomes of bariatric surgical procedures on macro and microvascular complications of T2DM. Patients underwent bariatric surgery (n=2,580) and control (nonbariatric surgery patients, n=13,371). After bariatric surgical procedures, the results showed microvascular events with adjusted HR 0.22, 95% CI 0.09 to 0.49, and macrovascular events with adjusted HR 0.39, 95% CI 0.29 to 0.51. Overall, there was a 65% reduction in the microvascular and macrovascular complications of T2DM after bariatric surgery (Figure 7).155
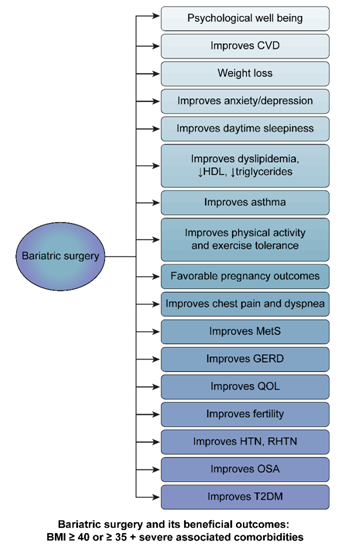
Figure 7 Bariatric surgery and its beneficial outcomes: BMI ≥40 or ≥35 severe associated comorbidities.
Another retrospective cohort study explored the effects of bariatric surgery on the older population; for patients who were <65, the survival rate advantage began six months after the surgical procedure, andafter 11 months for patients who were elderly compared to measurements from the control group (P<0.001) over two years of follow up period. Also, these patients showed a marked improvement in their associated comorbidities, such as OSA, HTN, T2DM, dyslipidemia, and CVD (P<0.001), after one year of the surgical period compared to the control group (nonsurgical).156 Bariatric surgery is also very effective in reducing musculoskeletal pain as compared to conventional treatment of weight loss in severely obese patients.157
Obesity is a worldwide epidemic (Wilson et al, 2002; McTigue et al, 2006). Obesity risk factors are decreased physical activity, sedentary lifestyles—such as watching T.V. for a prolonged time period, playing video games, and excessive use of computers—and eating unhealthily, consuming junk and fast food and drinking a lot of sugary drinks. Affluent socioeconomic status and family history of obesity are contributing factors in obesity epidemiology (Mistry et al, 2015).Obesity rates increase with age, because as an individual age, his or her physical activity decreases. Obesity increases the risk for the components of metabolic syndrome including HTN and T2DM, which can lead to CVD and increase the rate of mortality.158,159 The weight loss measure could be focused on dietary intake and physical activity (Haslam et al, 2005). Diet and exercise counseling helped patients to lose 3-4 kg in 1-3.3years. Additionally, these measures improved general physical functioning and glucose tolerance, and they decreased the incidence of T2DM, HTN, and CVD.
In young adults, disturbed sleep management should be addressed, along with stress and depression management. Keeping in mind that attention should also be placed on emotional issues, involving a person in healthy group discussions and therapies, encouraging them to actively participate in these groups, can strengthen psychological, mental, and social health. Health care workers, especially primary care physicians, can play a vital role in solving this growing public health problem.160
In the management of obesity and its related comorbidities, there is a need to make strong policies nationwide as well as globally, not only to prevent but also to manage this rising global health issue.161 The obesity diagnosis and management plan should be discussed and encouraged at office visits with the primary care physician, as most of the obese patients do not have the official diagnosis of obesity; therefore, their plan formulation regarding addressing obesity suffers.162 In a primary care setting, if the patient’s BMI is ≥30, the patient should be referred to weight management programs.
Weight management in obese and overweight individuals is a challenging and long-term, continuous process which initially includes lifestyle modifications such as adapting a low caloric diet, engaging in daily physical exercises, and beginning behavioral treatments as consuming a low energy diet and doing more physical exercise.163 Pharmacological treatments should be given if the BMI > 30 or > 27 with comorbidities.164 Bariatric surgical procedures are safe, even in the elderly population, so this should not be a deterrent to undergo the operations.165 Overweight/obese patients carry a huge health and economic burden by decreasing individual productivity due to disabilities and early death. The treatment of weight loss and associated comorbidities such as T2DM, hyperlipidemia, HTN, and CVD can be moderately reduced but will still increase the total cost significantly.166
Obese patients who are candidates for bariatric surgery should go for surgical intervention at the earliest possible time in order to achieve the best outcomes. As the study shows, patients with a shorter duration of diabetes (less than 5-10 years) or those on oral agents had a nearly two-fold greater chance of having a resolution of their T2DM than those who had the disease greater than 10 years or those taking insulin.135 In a meta-analysis of 136 studies (n=22,094) which looked at co-morbid health outcomes in those who had bariatric surgery for weight loss, the outcomes of bariatric surgery were: T2DM, completely resolved in 76.8% of patients and improved in 86.0% of patients. HTN, resolved in 61.7% of patients and improved in 78.5% of patients. Hyperlipidemia was improved in 70% of patients, and OSA was resolved in 85.7% of patients and improved in 83.6% of patients.167
Obesity is also one of the main independent risk factors of CVD; in 2004, the American College of Cardiology Foundation endorsed and provided a full treatment and management guideline approach to cardiologists and related health care professionals. Weight loss and physical activity are the basic key factors to reduce CVD.168–196
This review highlights the impact of obesity on its associated comorbidities. Indeed, in several populations across different cultures, the positive relationship between obesity and all-cause mortality is pronounced. This represents an important public health crisis that demands multi-layered interventions. To cope with this increasing and seriously preventable health issue, we emphasize the need to minimize the consumption of junk and fast food, increasing the consumption of fresh fruits and vegetables. Other important habits include drinking water instead of juice, soda, or alcohol as well as smoking cessation. In addition to making dietary changes, engaging in physical exercise is necessary. If medical and/or lifestyle medication therapies fail, then surgical interventions are another option to treat obesity and its associated comorbidities. Surgical procedures which can drastically reduce obesity and its related comorbidities ultimately improve mortality rates. To improve public health, obesity and its associated comorbidities should be seriously considered. Solving this issue is not only the responsibility of an individual and a healthcare worker, but it should be properly addressed by government officials to improve the health of individuals and make an overall healthier society.
This work is supported by the following funding agencies: R25-HL105444 and R25-HL 116378 (NHLBI); R01-MD007716 (NIMHD) to GJL. However, the funders had no role in study design, data collection, analysis, or decision to publish.
The author declares that there are no conflicts of interest.
None.

©2020 Jehan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.