Advances in
eISSN: 2378-3168


Research Article Volume 9 Issue 2
1Sri Bhadrakali Diabetic Clinic, India
2Department of Clinical Pharmacy & Pharm D, Vaagdevi College of Pharmacy, India
3Rakshith Multispeciality Hospital, India
Correspondence: Bandaru Shesagiri Sharvana bhava, Associate professor, Department of Clinical Pharmacy & Pharm D, Vaagdevi College of Pharmacy, Warangal, Telangana, India
Received: March 13, 2019 | Published: April 16, 2019
Citation: Siva SB, Akhil D, Haripriya K, et al. Efficacy of telmisartan and enalapril in patients with diabetic nephropathy. Adv Obes Weight Manag Control. 2019;9(2):53-57. DOI: 10.15406/aowmc.2019.09.00274
Introduction: Diabetic nephropathy is characterized by hypertension and persistent proteinuria and is the leading cause of end stage renal disease (ESRD). The comparison of Telmisartan and Enalapril was designed to assess and compare the efficacies of both drugs in diabetic nephropathy patients.
Material and methods: All age groups of patients diagnosed with diabetic nephropathy are included in our study.
Results: 112 patients were recruited in the study. Patients taking Telmisartan and Enalapril once daily completed the study. There was a significant reduction in urine albumin, urine creatinine, urine albumin/creatinine ratio (UACR), serum creatinine, Blood pressure, Fasting blood sugar, Post lunch blood sugar, HbA1C, Total cholesterol, low density lipoprotein, very low density lipoprotein, high density lipoprotein and triglycerides.
Conclusion: Both Telmisartan and Enalapril were efficacious in diabetic nephropathy patients, but Enalapril showed more Reno protection than Telmisartan in this study.
Keywords: diabetic nephropathy, ESRD, telmisartan, enalapril, proteinuria
Diabetes mellitus is a metabolic complex disorder characterised by hyperglycaemia and glucose intolerance as their hallmark due to insulin deficiency or impaired effectiveness of insulin action.1 Diabetic nephropathy is one of the potential micro vascular complications in diabetic patients. It is the leading cause of End stage renal disease (ESRD). Diabetic kidney disease refers to chronic kidney disease (CKD) presumed to be caused by diabetes.2 Diabetic nephropathy is screened for persistent abnormal urine albumin excretion and by decreased glomerular filtration rate (GFR). Albuminuria has been divided into micro albuminuria (urine albumin creatinine ratio (UACR) 30-300 mg/dl) and macro albuminuria (UACR more than 300mg/dl). Serum creatinine derives estimates of GRF and diabetic kidney disease.3
Diabetic kidney disease can be detected by screening for persistent abnormal urine albumin excretion and by determining the estimated glomerular filtration rate. The main evidence based strategies for preventing or delaying loss of kidney function in diabetic patients include blood pressure control, blockade of renin-angiotensin system, and glycaemic control. Controlling these factors and reducing proteinuria are now the main focus of diabetic kidney disease management. Through a multidisciplinary approach of implementing guidelines and timely referral, care of the diabetic kidney disease patient can be improved. The key is preventing and slowing the progression of this complication, to keep the other shoe from dropping.4,5
The aim of the study is to assess and compare the efficacy of Telmisartan and Enalapril in diabetic nephropathic patients. Angiotensin converting enzyme inhibitors and angiotensin receptor blocker have reno protection effects in diabetic patients. Enalapril; Angiotensin-converting–enzyme (ACE) inhibitors, which competitively block the renin–angiotensin system, decrease glomerular capillary pressure and prevent the progression of microalbuminuria to overt proteinuria.6 The side effects of enalapril are Edema, Dry cough, Dizziness, Hypertension, Syncope. Enalapril is contraindicated in pregnancy and breast feeding. Telmisartan is a angiotensin receptor antagonist possessing selective, and insurmountable inhibitory activity specific to the angiotensin П type 1 (AT1) Receptor.7 side effects are Tachycardia, Bradycardia, Hypotension, Edema and Allergic reactions. Telmisartan is contraindicated during pregnancy, in bilateral renal artery stenosis in which it can cause renal failure.
Literature
(Table 1).8–11
|
Author |
Year |
Study |
Conclusion |
|
Roland et al.8 |
2013 |
Telmisartan in incipient and overt diabetic renal disease. |
The Effect of telmisartan on kidney function support its use in patients with microalbuminuria or overt diabetic nephropathy. |
|
Bhansali et al.9 |
2010 |
Antialbumineric efficacy of ACE inhibitors and ARB’S in type 1 DM with nephropathy. |
Dual blockade with ramipril enhanced with antialbumineric effect of telmisartan and reduced in blood pressure. |
|
Anthony et al.10 |
2004 |
ARB’S versus ACE inhibitors in type 2 DM and nephropathy. |
Telmisartan is not inferior to enalapril in providing long term reno protection in persons with type 2 diabetes mellitus. |
|
Johnsen et al.11 |
1992 |
Renal protective effect of enalapril in diabetic nephropathy |
Treatment with enalapril can reduce the rate of decline in kidney function in patients with diabetic nephropathy. |
It is a prospective and observational research done in “SRI BHADRAKALI DIABETIC CLINIC” Naimnagar, Hanamakonda and Rakshith Multispeciality Hospital, Kakaji Colony, Warangal, Telangana, India. Patients were explained about the study & informed consent forms were seeked by explaining them in their local language. Institutional Human Ethical Committee Endorsement was obtained after submission of protocol and IHEC NO. is MGM/VCOP/PHARMD/V/011/2018.
Inclusion criteria
Exclusion criteria
Study design
It is a prospective, observational, comparative study design, and the patients who are taking Telmisartan and Enalapril were included.
Clinical response assessment
The efficacy of Telmisartan and Enalapril was assessed by measuring the change in urine micro albuminuria, serum creatinine, Blood pressure, fasting blood sugar, post lunch blood sugar, HbA1C, Total cholesterol, low density lipoprotein, very low density lipoprotein, high density lipoprotein and triglycerides after 12weeks of treatment. Primary end point was change in urine albuminuria, urine creatinine, urine albumin/creatinine ratio (UACR) and serum creatinine. Secondary end point was change in blood pressure, fasting blood sugar, post lunch blood sugar, HbA1C, total cholesterol, high density lipoprotein, very low density lipoprotein, low density lipoprotein, and triglycerides were measured at 12 weeks compared to the baseline levels.
Statistical analysis
All the parameters were expressed as mean±SD was performed by using the MS-EXCEL sheet 2013. All the parameters were obtained at baseline at the end of study.
(Figures 1–15).
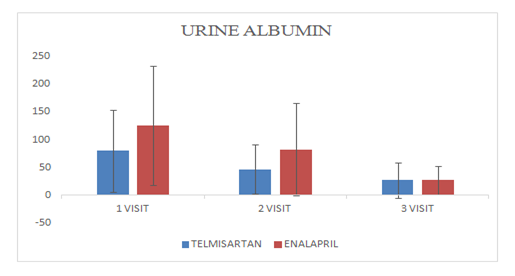
Mean change of urine albumin in Telmisartan group was 27.09±31.56
Mean change of urine albumin in Enalapril group was 27.09±25.70
Figure 1 Distribution of patients.
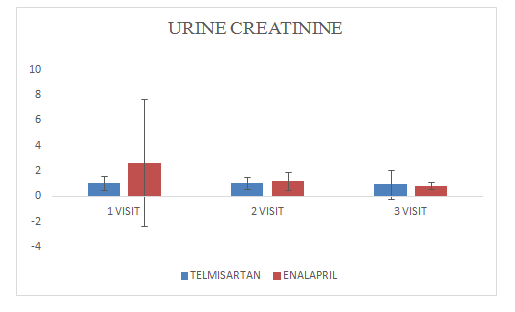
Mean change of urine creatinine in Telmisartan group was 0.94±0.4
Mean change of urine creatinine in Enalapril group was 0.83±0.26
Figure 2 Urine Albumin.
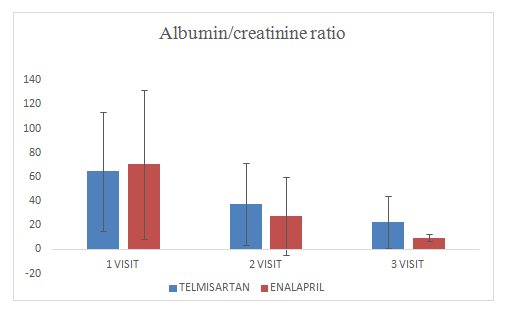
Mean change of urine albumin/creatinine ratio in Telmisartan group was 22.89±21.3
Mean change of urine albumin/creatinine ratio in Enalapril group was 9.7±3.21
Figure 3 Urine Creatinine.
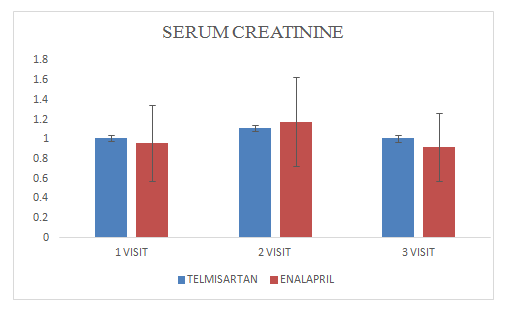
Mean change of serum creatinine in Telmisartan group was 1.0±1.17
Mean change of serum creatinine in Enalapril group was 0.09±0.34.
Figure 4 Urine albumin/creatinine ratio (uacr).
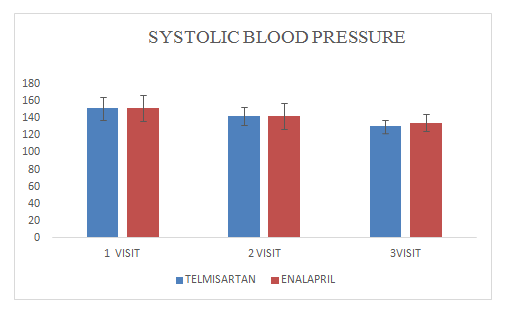
Mean change of systolic blood pressure in Telmisartan group was 130.64±7.6
Mean change of systolic blood pressure in Enalapril group was 134.54±9.98
Figure 5 Serum Creatinine.
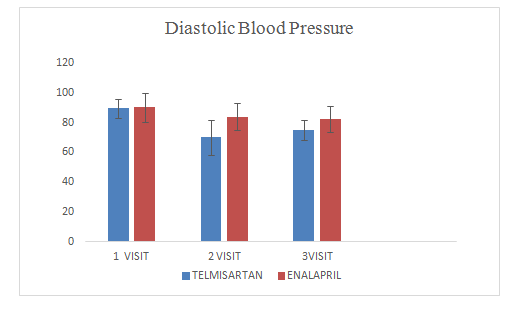
Mean change of diastolic blood pressure in Telmisartan group was 75.65±9.98
Mean change of diastolic blood pressure in Enalapril group was 82.5±8.94
Figure 6 Systolic Blood Pressure.
Mean change offasting blood sugar in Telmisartan group was 128.29±43.07
Mean change offasting blood sugar in Enalapril group was 122.63±30.90
Figure 7 Diastolic blood pressure.

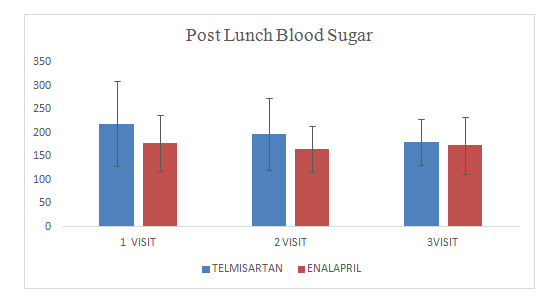
Mean change of post lunch blood sugar in Telmisartan group was180.4± 48.90
Mean change of post lunch blood sugar in Enalapril group was 173± 59.90
Figure 8 Fasting Blood Sugar.
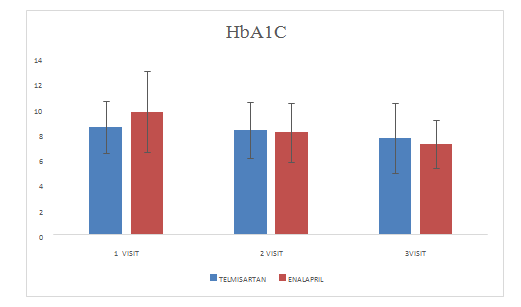
Mean change of HbA1Cin Telmisartan group was 7.6±2.80
Mean change of HbA1Cin Enalapril group was 7.19±1.89
Figure 9 post lunch blood sugar.
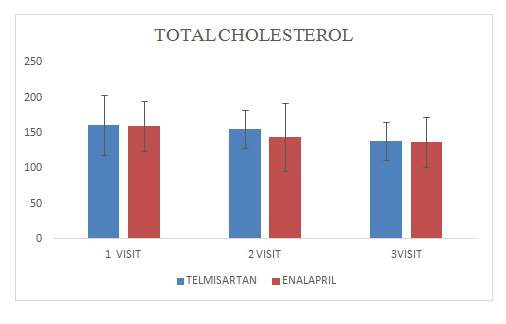
Mean change of total cholesterol in Telmisartan group was 138.27±27.04
Mean change of total cholesterol in Enalapril group was 137.12±35.54
Figure 10 HbA1C.
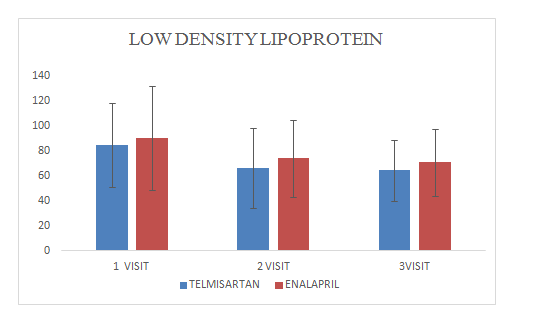
Mean change of low density lipoprotein in Telmisartan group was 64.29±24.4
Mean change of low density lipoprotein in Enalapril group was 70.8±26.64
Figure 11 Total cholesterol.
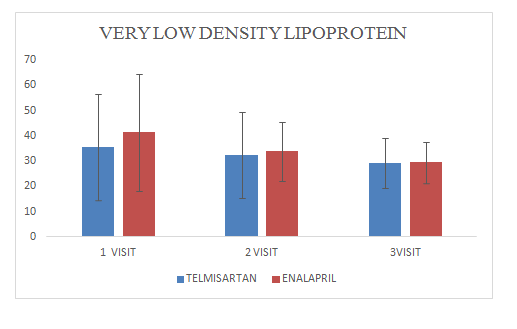
Mean change of very low density lipoproteinin Telmisartan group was 29.33±9.7
Mean change of very low density lipoprotein in Enalapril group was 29.5±8.11
Figure 12 Low Density Lipoprotein.
Mean change of high density lipoproteinin Telmisartan group was 35.20±14.7
Mean change of high density lipoproteinin Enalapril group was 31.68±6.25
Figure 13 Very Low Density Lipoprotein.
Comparision of efficacy of Telmisartan and Enlapril was done in patients with diabetic nephropathy. The following glycemic parametresblood pressuresystemic blood pressure,diastolicfasting blood sugar, post lunch blood sugar,HbA1C ,micro albuminuria (urine albumin, urine creatinine, urine albumin /creatinine ratio [UACR]),total cholesterol, high density lipoprotein, very low density lipoprotein, low density lipoprotein, and triglycerides were obsereved. To our knowledge, there has been one clinical study that has directly compared the effect of an angiotensin II–receptor blocker (losartan) with that of an ACE inhibitor (enalapril) in subjects with type 2 diabetes and early nephropathy.13 That short-term study indicated that both drugs reduced urinary albumin excretion; differences between the treatments were not significant. In our study Telmisartan and Enalapril therapy from baseline to visit-3 has shown a greater efficacy in both groups but enlapril group is more efficacious in decreaseing the microalbuminuria levels than telmisartan group.
Both Telmisartan and Enalapril were efficacious in diabetic nephropathy patients, but Enalapril confers more reno protection when compared with Telmisartan. This study helps Clinicians in making selection of an effective drug between an ACE inhibitor and an ARB, but Clinicians should consider side effects and contraindications while prescribing these drugs in diabetic nephropathy patients.
None.
Author declares there is no conflict of interest.

©2019 Siva, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.