Advances in
eISSN: 2378-3168


Perspective Volume 7 Issue 3
1Department of Health and Nutrition, Asia University, Taiwan
2Internal Medicine, China Medical University, Taiwan
Correspondence: Henry J Tsai, Department of Health and Nutrition, Asia University, Taiwan
Received: August 28, 2017 | Published: September 12, 2017
Citation: Tsai HJ, Chen CC. A new perspective on appetite control: protein tyrosine phosphatase 1b (ptp1b) inactivation by oxidative phytochemicals. Adv Obes Weight Manag Control. 2017;7(3):285-287. DOI: 10.15406/aowmc.2017.07.00197
Obesity and overweight are resulted from poor control on appetite. A major part of appetite control relies on leptin and its receptor signal pathway (Figure 1). One obvious evidence supporting this idea can be observed from the mutation on leptin (ob gene in obese mice) or leptin receptor (db gene in diabetic mice). C57Bl mice with ob/ob gene will develop obesity while mice with db/db gene will develop obesity and diabetes. The recessive ob gene mutation is due to an early termination on the leptin gene transcript.1 A replacement therapy on ob/ob mice with full length leptin resulted in reduced appetite and reduction in body weight.2
The leptin signal pathway relies on Janus kinase (JAK) and signal transducer/activator of transcription (STAT) proteins to propagate its signal. When leptin binds to its receptor, JAK2 is activated to phosphorylate leptin receptor and STAT3. The phosphorylated STAT3 dimerize and become capable of modulating gene expression in the nucleus. The JAK/STAT signal is modulated by a negative regulator, protein tyrosine phosphatase 1b (PTP1B), which dephosphorylates STAT3 and thus renders STAT3 back to its ground state.3,4 Incidentally, insulin signal is also modulated by PTP1B. Insulin receptor activation results in phosphorylation on insulin receptor itself and its substrate, insulin receptor substrate (IRS). Both insulin receptor and IRS are modulated by PTP1B. Therefore, a potent PTP1B inhibitor has been proposed as a dual function inhibitor for treating obesity (leptin pathway) and diabetes (insulin pathway), as illustrated in Figure 2.
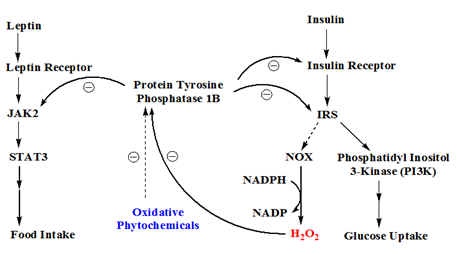
Figure 2 The role of protein tyrosine phosphatase 1b in leptin and insulin signal pathways. Leptin signal is passed on by JAK2 and STAT3, while insulin signal is mediated by insulin receptor substrate (IRS). IRS can in turn activate phosphatidyl inositol 3-kinase (PI3K) leading to enhanced glucose uptake and/or upregulated NADPH oxidase (NOX) which leads to an increase of hydrogen peroxide formation. PTP1B is a negative regulator which dephosphorylates JAK2 in leptin signal pathway or insulin receptor/IRS in insulin signal pathway. The endogenous H2O2 can inactivate protein tyrosine phosphatase 1b (PTP1B) transiently. Some oxidative phytochemicals can augment the oxidative potential of H2O2.
Activated insulin receptor can provoke several different pathways. One interesting but still poorly understood pathway is the activation of NADPH oxidase (NOX) which results in an increase in oxygen superoxide.5,6 Oxygen superoxide is then converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). It has been shown that endogenous H2O2, arisen from insulin stimulation, can inactivate PTP1B, which in turns potentiates insulin signal transiently,7,8 as illustrated in Figure 2.

Figure 2 The role of protein tyrosine phosphatase 1b in leptin and insulin signal pathways. Leptin signal is passed on by JAK2 and STAT3, while insulin signal is mediated by insulin receptor substrate (IRS). IRS can in turn activate phosphatidyl inositol 3-kinase (PI3K) leading to enhanced glucose uptake and/or upregulated NADPH oxidase (NOX) which leads to an increase of hydrogen peroxide formation. PTP1B is a negative regulator which dephosphorylates JAK2 in leptin signal pathway or insulin receptor/IRS in insulin signal pathway. The endogenous H2O2 can inactivate protein tyrosine phosphatase 1b (PTP1B) transiently. Some oxidative phytochemicals can augment the oxidative potential of H2O2.
PTP1B is a phosphoryl hydrolase catalyzed by a cysteine residue. During the catalysis, the catalytic cysteine is deprotonated to thiolate anion which becomes a nucleophile. The cysteine thiolate anion is susceptible to H2O2 oxidation to sulphenic acid (-SOH) which renders PTP1B inactive.9,10 The sulphenic group on cysteine will condense with its adjacent amine group of n+1 peptide residue to become a sulphenylamide, resulting in an isothiazolidine ring on the peptide backbone.11 At the sulphenylamide stage, PTP1B enzyme activity can be restored by thiol reducing agents, e.g., glutathione or dithiothreitol, to its active thiolate anion stage. Thus, a mild oxidation on the catalytic cysteine of PTP1B is considered reversible, although the oxidation itself involves formation of covalent bonds. If the sulphenic group on PTP1B is further oxidized to sulphinic acid (-SO2H) or sulphonic acid (-SO3H), PTP1B enzyme activity is lost irreversibly, as illustrated in Figure 3.
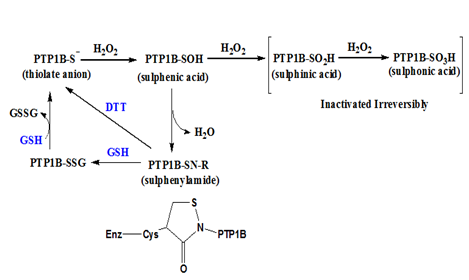
Figure 3 Reversible and irreversible oxidation of cysteine thiol on PTP1B. The thiol or thiolate anion of catalytic cysteine on PTP1B can be oxidized to a sulphenic acid form which can condense with an amine group to form a sulphenylamide resulting in an isothiazolidine ring on the peptide backbone. The sulphenylamide can be reduced by thiol reducing agents like dithiothreitol (DTT) or glutathione (GSH). If the sulphenic acid is further oxidized to sulphinic acid or sulphonic acid, they cannot be reduced back to the thiol form in vivo.
Since the PTP1B gene knocked out mice was published in 1999,12 many scientists have been seeking a potent PTP1B inhibitor, which in theory can treat diabetes, overweight or even certain form of her2+ breast cancer (her2 oncogene is also regulated by PTP1B).13 The difficult part of finding a satisfactory PTP1B inhibitor is because T cell PTP (TCPTP) isozyme bears a very similar active site contour. So PTP1B inhibitors are not able to distinguish these two PTP isozymes apart.14,15 As TCPTP is important for maintaining immune system function, mice lack TCPTP will suffer immune deficiency or even lethality.16 It is paramount to inhibit PTP1B but not TCPTP. For example, a recent PTP1B inhibitor17 with a selectivity of 30-fold over TCPTP is not good enough for pharmaceutical purpose, because pharmaceutical reagents require an isozyme selectivity of 1000 fold or greater (>3-order difference in IC50) to be considered safe.
Since PTP1B can be inactivated transiently by endogenous H2O2, while PTP1B specific inhibitors are not ready for medical use, an alternative approach of inhibiting PTP1B may be using phytochemicals to mimic the action of H2O2. What compounds possess such oxidative power as H2O2? Isothiocyanate compounds found in cruciales are capable of inactivating PTP1B.18 More recently, guava leaf extract from our laboratory suggest that polar phytochemicals in the guava leaf extract are also capable of inactivating PTP1B enzyme with stronger reactivity (20-80 folds higher pseudo-first reaction rates than those of isothiocyanates). While the identities of these compounds have not been identified, they do show a hypoglycemic efficacy when fed to healthy mice as well as a supplementary effect to insulin stimulated glucose uptake in adipocytes (presumably through an inactivation on PTP1B but elucidated on the insulin pathway). These results would suggest guava leaf extract, i.e., guava leaf tea, may have an appetite suppressing effect because of its oxidative potential on PTP1B.
None.
The author declares no conflict of interest.

©2017 Tsai, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.