eISSN: 2379-6367


Literature Review Volume 10 Issue 1
1Department of Pharmacology, Cerrahpasa Faculty of Medicine, Istanbul University, Turkey
2Department of Medical Pharmacology, Faculty of Medicine, Beykent University, Turkey
3Department of Internal Medicine, Okmeydani Training and Research Hospital, Turkey
4Department of Pharmaceutical Chemistry, School of Pharmacy, Istanbul Medipol University, Turkey
5Department of Pharmacology, Faculty of Pharmacy, Hacettepe University, Sihhiye, Ankara Turkey
Correspondence: Gokhan Faikoglu, Department of Medical Pharmacology, Faculty of Medicine, Beykent University, Istanbul, Turkey
Received: January 27, 2022 | Published: February 18, 2022
Citation: Saygisever-Faikoglu K, Faikoglu G, Ozcan FO, et al. The efficacy and safety of fenticonazole in the treatment of mixed vaginitis. Pharm Pharmacol Int J. 2022;10(1):12-20. DOI: 10.15406/ppij.2022.10.00358
Background: Vaginitis means inflammation of the vaginal mucosa and is one of the most common causes for consulting to a gynecologists. Mixed-type vaginal infections are simultaneous infections of at least two types of vaginal pathogens (for example, a bacteria and a Candida species). In the treatment of mixed vaginitis, broad spectrum antimicrobial therapy effective on different kinds of pathogens is required.
Aim: The purpose of this review is to evaluate the place and efficacy of fenticonazole nitrate (2% vaginal cream and 600 and 1000 mg ovule forms) in mixed-type vaginitis.
Method: Literatures were retrieved by a PubMed search, using different combinations of pertinent keywords (e.g., fenticonazole, fungal infection, mixed vaginal infections), without any limitations in terms of publication date and language. Papers which assessed the therapeutic efficacy and tolerability of fenticonazole in patients with mixed vaginitis were selected for inclusion according to their relevance for the topic, as judged by the authors.
Results: Fenticonazole has a broad spectrum of activity, including antifungal, antibacterial and antiparasidic effects. Fenticonazole nitrate provides significant improvement in symptoms such as vaginal discharge, pruritus and burning associated with candidal vulvovaginitis, with a cure rate of 97.5%. In addition, while fenticonazole provides 85% cure rates in bacterial vaginitis, it is an effective topical option with a clinical cure rate of 87% in mixed vulvovaginal infections with Candida albicans, Gardnerella vaginalis and/or Trichomonas vaginalis. Fenticonazole provides up to 90% improvement in symptoms such as pruritus, burning, redness, discharge and edema, which are frequently observed in vaginitis, with its rapid onset of action and excellent tolerability from the first days of treatment.
Conclusion: Fenticonazole has a broad spectrum of antimicrobial activity with its antifungal, antibacterial and antiparasidic effects. It is an ideal topical alternative with its high clinical efficacy in Candidal vulvovaginitis caused by Candida albicans, bacterial vaginosis caused by Gardnerella vaginalis, gram (+) and/or gram (-) bacteria, and Trichomonal vaginitis caused by Trichomonas vaginalis and mixed vaginitis caused by these pathogens and its excellent tolerability. Fenticonazole has lower efficacy against the three main lactobacillus strains called Lactobacillus gasseri, Lactobacillus jensenii and Lactobacillus crispatus found on the vaginal flora compared to miconazole and metronidazole with high MID rates. Fenticonazole is a treatment option with efficacy, safety and excellent tolerability that can be the first choice empirically in the first-line treatment of mixed-type vaginitis, which has been shown to be effective in fungal, bacterial and mixed-type vaginal infections with its broad spectrum of action in various clinical studies.
Keywords: fenticonazole, bacterial vaginitis, mixed vaginitis, vulvovaginal candidiasis
MID, minimum ınhibitory dilution; MIC, minimum ınhibitory concentration; SAP, secretory acid proteinase; RNA, ribonucleic acid; EUCAST, european committee on antimicrobial susceptibility testing; CLSI, clinical and laboratory standards ınstitute
Vaginitis is defined as inflammation of the vaginal mucosa as a result of dysfunction of the vaginal flora and is the most common reason for consulting to the gynecologists.1 Vaginitis is characterized by inflammation-related complaints accompanied by erythema, pruritus, vaginal discharge and edema. Conditions such as hormonal changes in the vaginal epithelium, increase in vaginal pH from 3.5-4.5 to 6-8, urinary and/or fecal incontinences, atrophy in the vaginal tissue, trauma in the prepubertal, pubertal or postmenopausal periods cause the vagina to become open to infection and as a result, vaginitis develops by causing the lactobacilli in the flora to leave their places to the mixed flora, predominantly to pathogenic cocci.2
Three groups of pathogens are responsible for the formation of vaginitis, mostly (90%) fungi (eg, Candida albicans), bacteria (eg, Gardnerella vaginalis), parasites (eg, Trichomonas vaginalis). Vaginitis can be observed in 90% of women at least once in their lives, and the association of two or more types of pathogens is observed in approximately 30% of the cases.3-5 Vaginal infection with at least two of the bacterial and/or fungal agents is called mixed vaginitis. Although mixed vaginitis can be treated with treatment approaches that combine antifungal and antibacterial agents, this approach brings with it disadvantages in terms of increased resistance development and/or systemic and/or local side effects related to this treatment algorithm.6,7 This makes molecules with proven multiple effects, such as fenticonazole, more valuable.8
Fenticonazole [α-(2,4-dichlorophenyl)-β, N-imidazolylethyl 4-phenylthiobenzyl ether nitrate], an imidazole derivative molecule, was discovered in 1973 for the topical treatment of dermatophytes and fungal infections and licensed in 1986. It is an effective and well tolerated broad-spectrum antimicrobial used in the topical treatment of skin and vulvovaginal tissue.9-11
The antifungal activity of fenticonazole has been proven in vitro and it shows this activity against cutaneous-mucous membrane mycoses:12
The mechanisms of action of fenticonazole nitrate against Candida species are listed below;13
Fenticonazole also has an antibacterial effect against gram (+) and gram (-) bacteria, and the mechanism of action of this activity is different from other antibiotics. It shows its effect at the membrane level by increasing membrane permeability, at the cytoplasmic level by inhibiting the oxidative process in mitochondria, and at the nuclear level by inhibiting RNA synthesis.12,14 In addition, it prevents the reproduction of bacteria and protozoa (Trichomonas species) by causing inhibition of the pyruvate oxido-reductase enzyme, which is necessary for cell division or proliferation.13,14
Fenticonazole has a unique spectrum of action that is antibacterial against gram (+) and gram (-) bacteria (Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus spp, Escherichia coli) and Gardnerella Vaginalis, which super-infect the skin and cause vaginal infections, and antiparasitic against Trichomonas vaginalis.14,15 Its anti-Trichomonas activity is dependent on a radical anion with cyto-lethal activity with a short half-life produced under anaerobic conditions, and its antibacterial activity depends on its selective cytotoxic oxidative metabolite.14,16
Fenticonazole nitrate vaginal capsule dissolves after contact with vaginal fluid and rapidly penetrates into the vaginal mucosa where Candida aggregation is present. It remains active in the mucosa for a long time, and it has been reported that the remaining part in the vaginal fluid is also active.17 Fenticonazole nitrate has a dose-dependent inhibitory effect on the acid proteinase enzyme secreted by Candida albicans. This inhibition is seen even at low concentrations of fenticonazole which are not sufficient to inhibit Candida growth. This feature specific to fenticonazole is not seen in ketoconazole, fluconazole, miconazole and econazole.17
Pharmacokinetic studies have shown that there is no subcutaneous absorption of fenticonazole in either women or animals, and the vaginal absorption is very low.12 Fenticonazole applied on the skin remains in the stratum corneum for 72 hours; this ensures that the active form of the drug, which does not require reapplication, is stored in the skin as a reservoir and shows its effectiveness. However, very little of the drug stored in the skin and vagina passes into the systemic circulation.12,18 After vaginal administration of fenticonazole, its effectiveness has been demonstrated in the vaginal mucosa for 72 hours and in plasma for 96 hours18,19 Fenticonazole has no effect on the release of histamine, adrenaline, noradrenaline and acetylcholine, and on blood pressure, heart rate and pulmonary respiration.20
In vitro studies focused on the mixed mycotic and bacterial activity of fenticonazole in fungal skin infections superinfected by S. aureus, coryneforms and streptococci. The antibacterial activities of fenticonazole on Bacteroides isolates (B. melaninogenicus-B. oralis group, G. vaginalis, Mobiluncus spp. and anaerobic, gram (+) cocci) which are commonly associated with vaginosis have also been shown in in vitro studies.15
In the study in which antibiotic susceptibility tests (European Committee on Antimicrobial Susceptibility Testing; EUCAST) and Clinical and Laboratory Standards Institute (CLSI) for fenticonazole, fluconazole, ketoconazole and itroconazole performed on isolates taken from 260 women diagnosed with vulvuvaginal candidiasis, the antibiotic susceptibilities of C. albicans and C. glabrata were tested. When the Minimum Inhibitory Concentrations (MIC; antimicrobial concentration that inhibits microbial growth by 50% or more) of antibiotics were evaluated, the MIC value of fenticonazole was found to be lower than other drugs.21
In another study, the efficacy and antibacterial activity of fenticonazole on Candida species were evaluated in 318 vaginitis isolates in vitro. MIC for efficacy and time passed for death of pathogens (t50, t90, t99 and t99.9) were evaluated as time-kill data. Fenticonazole showed activity against all species, but the MIC value measured for S. aureus was found to be higher than that of E.coli, S. agalactiae, C. albicans and other Candida isolates. The lowest MIC values were measured in S. agalactiae, C. parapsilosis, and G. vaginalis isolates. The strains, MIC values and time kill durations (t99,9) in which fenticonazole achieved a 99,9% kill rate are as follows: 10 hours for Staphylococcus aureus, Streptococcus agalactiae and Escherichia coli, 17 hours for C. albicans and Candida parapsilosis at 4XMIC; 19 hours for Candida glabrata, 20 hours for Candida tropicalis at 8XMIC; 17 hours for pure C. albicans and 20 hours for mixed cultures of C. albicans with S. aureus, S. agalactiae, or E. coli at 2XMIC. Supra-MIC values are achievable in the vaginal regions of patients treated topically.16 The MIC values obtained in this study highlight the efficacy of fenticonazole against Candida and bacterial species, and the killing time data highlight the microbicidal potential of fenticonazole, which can reach supra-MIC values on the skin/mucous surfaces of patients treated with topical fenticonazole. These data show that the effect of fenticonazole on C. albicans, S. aureus and other mixed infectious agents in a concentration-dependent manner, shows that this drug is a suitable candidate molecule that can be used in vaginal infections of different etiologies.14 Indeed, although the focus of activity of fenticonazole is commonly observed as bacteria causing symptomatic vaginal discharge, intravaginal administration of this drug has also demonstrated efficacy in patients with candida vaginitis, bacterial vaginitis, or mixed vaginitis. With these properties, fenticonazole is an ideal topical alternative that can be preferred in the treatment of mixed, bacterial and fungal vaginitis.16
Azole-resistant isolates are responsible for particularly persistent and recurrent vaginal infections. The effect of fenticonazole on azole-resistant isolates was also evaluated in studies. The mechanism of this resistance is low azole accumulation in the cell as a result of the increased activation of the pump that throws the drug out of the cell, and low binding of the drug to the enzyme as a result of the mutation in the primary target 14-α-lanosteroldemethylase enzyme. For this purpose, fenticonazole was tested on fluconazola resistant C. albicans (20 isolates) and C. glabrata (30 isolates) isolates. The molecular mechanism of 25 fluconazole-resistant (10 C. albicans and 15 C. glabrata) isolates was found to be upregulation of genes encoding the pump that ejects the drug [for C. albicans only], and a point mutation in ERG11 encoding the SNQ2 [for C. glabrata only] and/or 14-α-lanosteroldemethylase enzyme.22 Fenticonazole, unlike other triazoles (possibly due to its oxidative cytotoxic activity), not only treats mixed fungal and bacterial infections,19 but also can overcome Candida infections with antifungal resistance.23 In addition, it was determined that the MIC values of fenticonazole were lower than the MIC values of fluconazole and showed higher activity than fluconazole. Fenticonazole activity has been shown to be effective against Candida species independent of pump-mediated fluconazole resistance to eject the drug. Based on these findings, it can be said that fenticonazole may be an ideal treatment option with its strong antifungal effects, especially in the treatment of patients with recurrent or persistent vulvovaginal candidiasis.22
Antibacterial effect of fenticonazole nitrate was studied in vitro conditions on gram-positive and gram-negative bacteria by Veronese et al. In the study, Fenticonazole nitrate showed a significant and high antibacterial effect on all gram-positive bacteria and Clostrudium novyi examined.10
It was shown in the study of Fontana et al. made in 1990 that Fenticonazole nitrate had an inhibitory effect against Trichomonas vaginalis in vitro conditions as an antiprotozoal activity. It has been reported that trichomonocidal activity develops at 50 mg/L doses, and its lethal activity begins between the 3rd and 4th hour intervals.24
In 2019, the in vitro antibacterial effect of fenticonazole was examined by Sanguinetti et al. in samples taken from 318 candida and bacterial vaginitis isolations with single-pathogen and bi-pathogen mixed culture analyzes.14 As a measure of effectiveness, minimal inhibitory concentrations (MIC) and time taken for pathogens to die (T50, T90, T99 and T99,9) were determined as kill-time data in the study.14
In the study of Sanguinetti et al., firstly, MIC values were measured for vaginal Candida and bacterial isolates (E. coli, S. agalactiae, S. aureus, C. albicans, C. glabrata, C. tropicalis C. parapsilosis and G. vaginalis). While fenticonazole was found to be effective against all species, the MIC value measured for S. aureus was slightly higher than for E. coli, S. agalactiae, C. albicans and other Candida species. The lowest MIC values were measured in S. agalactiae, C. parapsilosis, and G. vaginalis species (MIC 90; 0.03 g/ml).14
In the other phase of the same study, the time taken for pathogens to die at different doses was examined. For this, both single and mixed cultures of pathogens were created. C. albicans + S. aureus, C. albicans + E. coli or C. albicans + S. agalactiae were selected for mixed cultures. The time taken for fenticonazole to kill 50%, 90%, 99%, and 99,9% of pathogens in cultures was measured separately for each dose (time kill durations), with MIC values of 2, 4, and 8 times. It is seen that the microbiocidal action times in mixed cultures do not exceed the microbiocidal times required for Candida alone (Table 1).14
|
|
t99% |
t99,9% |
||||
|
2 x MIC |
4 x MIC |
8 x MIC |
2 x MIC |
4 x MIC |
8 x MIC |
|
|
Pure Cultures |
||||||
|
Candida albicans |
11.56 |
11.31 |
12.85 |
17.34 |
16.95 |
19.28 |
|
Candida glabrata |
NA |
>48 |
12,39 |
NA |
>48 |
18.59 |
|
Candida parapsilosis |
48 |
11.53 |
12.41 |
48 |
17.3 |
18.62 |
|
Candida tropicalis |
NA |
NA |
13.61 |
NA |
NA |
20.41 |
|
Staphylococcus aureus |
8.06 |
7.06 |
6.27 |
12.1 |
10.6 |
9.41 |
|
Streptococcus agalactiae |
7.46 |
6.87 |
6.09 |
11.19 |
10.3 |
9.13 |
|
Escherichia coli |
7.43 |
6.87 |
6.26 |
11.15 |
10.31 |
9.4 |
|
Mixed Cultures |
||||||
|
Candida albicans + Staphylococcus aureus |
13.87 |
10.45 |
12.36 |
20.81 |
15.68 |
18.54 |
|
7.71 |
6.8 |
5.74 |
11.57 |
10.19 |
8.62 |
|
|
Candida albicans + Staphylococcus agalactiae |
13.48 |
12.39 |
11.79 |
20.22 |
18.58 |
17.68 |
|
- |
7.08 |
6.57 |
- |
10.62 |
9.86 |
|
|
Candida albicans + E. coli |
13.66 |
13.28 |
11.83 |
20.49 |
19.93 |
17.74 |
|
7.71 |
6.8 |
5.74 |
11.57 |
10.19 |
8.62 |
|
Table 1 Times during which fenticonazole MIC value multiples prevent pathogen growth by 99% or 99.9% (t %, hour) (Adapted from Sanguinetti et al.14)
In addition, the MIC values of fenticonazole for vaginal Candida and bacterial isolates (E. coli, S. agalactiae, S. aureus, C. albicans, C. glabrata, C. tropicalis C. parapsilosis and G. vaginalis) and the time taken for pathogens to die at different doses are examined. The results report strong in vitro efficacy of fenticonazole also in S. aureus, E. coli or S. agalactiae species, which are bacterial infection agents that can be seen together with genital candidiasis, according to MIC data.14 In the light of these data, it has been reported that fenticonazole may exhibit strong efficacy in mixed-type vaginal infections in addition to vaginal Candida infections.14
In their study published in 1989, Jones et al. investigated the effectiveness of Fenticonazole against 177 strains of bacteria species associated with bacterial vaginosis or skin infections by measuring in vitro MIC values.15 In the study, they compared the in vitro antibacterial activity of fenticonazole with clotrimazole, miconazole, tetracycline and metronidazole. Bacteroides melahinogenicus-B. oralis group, Gardnerella vaginalis, Mobiluncus species and anaerobic, gram-positive cocci associated with bacterial vaginosis, showed high sensitivity to fenticonazole, clotrimazole and miconazole. Researchers state that the in vitro antibacterial activity determined in this study may be useful in the treatment of mixed vaginal infections with Fenticonazole. It has also been emphasized that fenticonazole, which has both candidiasis and bacterial activity and offers the possibility of topical use, can be preferred in terms of side effects and safety compared to metronidazole, which requires systemic treatment.15
In a different study examining the antibacterial effect of fenticonazole nitrate under in vitro conditions, it was observed that it showed a significant and high antibacterial effect on all gram-positive bacteria and Clostrudium novyi.10 In the study conducted by Veronese et al., and Costa et al., they determined the high efficacy of fenticonazole on 51 strains with different species of Trichophyton, Microsporum, Epidermophyton, Aspergillus, Candida, Cryptococcus families.9,10
Costa et al.25, Cusumano et al.26 also determined the high efficacy of fenticonazole in different Candida species and at different concentrations. Cusumano et al. reported the mixed mycotic and bacterial efficacy of fenticonazole in fungal skin infections superinfected by S. aureus, coryneforms and streptococci.26 In addition, its antibacterial activities on Bacteroides isolates (B. melaninogenicus-B. oralis group, G. vaginalis, Mobiluncus spp. and anaerobic, gram (+) cocci), which are commonly associated with bacterial vaginosis, were also emphasized in Cusumano et al.26 Jones et al.,15 and Fontana et al.,24 showed that fenticonazole nitrate has antiprotozoal efficacy and inhibitory effect against Trichomonas vaginalis in vitro in 1990. It has been shown in the study of Fontana et al. that trichomonocidal activity of fenticonazole develops at 50 mg/L doses, and its lethal activity begins at the 3rd and 4th hour intervals.
Mixed vaginitis is a type of vaginitis caused by more than one pathogen and therefore requires special treatment for complete eradication. Although two or more pathogens have been identified, co-infection is when one pathogen causes the present vaginal symptoms and the other pathogen behaves asymptomatically.
There are many clinical studies in the literature on the effects of fenticonazole on mixed and co-infection vaginitis.
Fenticonazole has been studied clinically in the field of gynecology, in the treatment of candida vulvovaginitis,27-36 and mixed infections.32,37-39 In all studies, the diagnosis was made on the basis of clinical history, direct mycological examination, and culture.
Fenticonazole (600-1000 mg/day) provided effective treatment in 75-100% of patients with candidal vulvovaginitis, and eradication was achieved in 70-100% of patients with Candida spp. within a week.27-37,40 It has been reported that fenticonazole nitrate provides significant improvement in symptoms such as vaginal discharge, pruritus and burning associated with candidal vulvovaginitis, with a cure rate of 97,5%.41
In the study, in which the efficacy and tolerability of 600 mg fenticonazole was evaluated in 417 patients with vaginal candidiasis,32 symptomatic improvements on the patients on day 1, 7 and 28 of the treatment were observed, and it was also detected that there was a meaningful decrease in vaginal discharge and pruritus by day 7. In microbiological control with vaginal smear, it was observed that 92,3% (385 of 417 women) responded to treatment with fenticonazole. At the end of one week, treatment was repeated in 84 women due to persistence of symptoms and a positive smear test. At the end of day 28, 392 patients (94%) had complete recovery, only 1 patient had worsening of symptoms. Side effects were observed infrequently; mild redness of the vulva and vagina and mild itching observed on the first day of treatment were mostly noted.
In another study,35 a single daily dose of 600 mg vaginal fenticonazole was administered to 54 patients of childbearing age with acute vaginal candidiasis. A second dose of 600 mg fenticonazole capsules was administered to seven patients with acute complicated vulvovaginal candidiasis on day 4 of treatment. In the second phase of the study, 2 doses of intravaginal fenticonazole capsules were administered to 57 patients with acute vaginal candidiasis, on day 1 and 4. Response to treatment was recorded as 100% in the first phase of the study and 97% in the second phase.
In a prospective study,37 80 patients with vulvovaginal candidiasis were randomly given intravaginal fenticonazole (600 mg) tablets or oral fluconazole (150 mg), and 3 days later, 2 doses of azoles were administered for short-term treatment. Improvement was observed in 32 (80%) of 40 patients in the fenticonazole group and in 31 (77,5%) of 40 patients in the fluconazole group at day 30 of post-treatment. Vulvovaginal pruritus healed in a shorter time in the fenticonazole group than in the fluconazole group (2,3 days. 4,5 days, p=0.047).
In patients with Vaginal Trichomoniasis, where fenticonazole vaginal capsule was administered once daily at a dose of 600 mg or 1000 mg for 2 or 5 days, 100% eradication was observed at day 7. It was observed that Candida spp. were also eradicated in these patients. It has been observed that a single dose of 1000 mg fenticonazole is more effective than 600 mg.8,42,43
In a randomized, double-blind study, the highest antimicrobial therapy relative to placebo in the treatment of patients with bacterial vaginosis was observed with 5g daily application of a 2% cream formulation of fenticonazole for 7 days. The recurrence rate of the infection was 11,8% in the drug group and 50% in the placebo group.8,44 It is also reported that fenticonazole provides 85% cure rates in bacterial vaginitis.19
In a multicenter, prospective study,39 fenticonazole (1000 mg vaginal suppository given once daily; on days 1 and 3) was used in mixed vulvovaginal infections with Candida albicans, Trichomonas vaginalis, and/or Gardnerella vaginalis. On the day 8, the eradication rate of Candida albicans was found to be 90% (26 of 29 patients) and no recurrence was reported. The success rate was 73% for G.vaginalis and 77% for mixed infection. Significant improvement in signs and symptoms was achieved on day 8 (p<0.05). Similar results have been reported in patients with vulvovaginal candidiasis treated with fenticonazole.31,32,40 It has been reported that symptoms such as erythema, edema sypmtoms, and pruritus and burning resolved within a few days after the first application, while some or all symptoms were completely resolved in 52% to 100% of patients within a week.
It is known that fenticonazole has antibacterial activity against gram (+) bacteria and anti-parasidic activity against Trichomonas.24,45 In this context, administration of the vaginal capsule form of fenticonazole as a single dose of 600 mg or 1000 mg for 5 days or for 2 days eradicated all microorganisms on day 7 in 28% to 65% of the patients; Trichomonas vaginalis and Candida spp were also eradicated in all patients on day 7. A single dose of 1000 mg of fenticonazole was found to be more effective than a single dose of 600 mg (p<0.01).34,42,43
Administration of 600 mg single dose or 1000 mg fenticonazole on the day 1 and 3 to patients with mixed infections, provided total eradication in 45% of the patients in day 7 and 8;38,39 90-96% of patients were eradicated from Candida albicans and 67-70% from Trichomonas vaginalis.
Fenticonazole is well tolerated in all formulations. The most common side effect observed is burning, mostly short-lived and mild to moderate, observed in 1-7% of patients. The most common side effect seen with fenticonazole when administered intravaginally is a burning sensation. However, this symptom of vaginal yeast infections is usually present in patients before drug administration.24,26-40,44,45
In the study of Scalambrino et al., the effect of 1000 mg fenticonazole vaginal pessaries was investigated in 67 patients infected with Trichomonas vaginalis alone or with Candida species. The results of the study were determined that fenticonazole destroyed all organisms in 10-12 days without any side effects.34
Bukovski et al. studied the effect of a single dose of 600 mg fenticonazole ovule in 87 patients with mixed vaginal infections with Trichomonas, Candida, and other pathogens. After 7 days of treatment, Trichomonas vaginalis was not found in 67% of the cases and Candida albicans was not found in 96% of the cases. The overall recovery rate was 87% (Figure 1). It was determined that the infection was completely cured when a second single dose treatment was administered to the patients who did not heal on day 7 and when they were called for control 4 weeks later. No systemic or local side effects have been reported.38
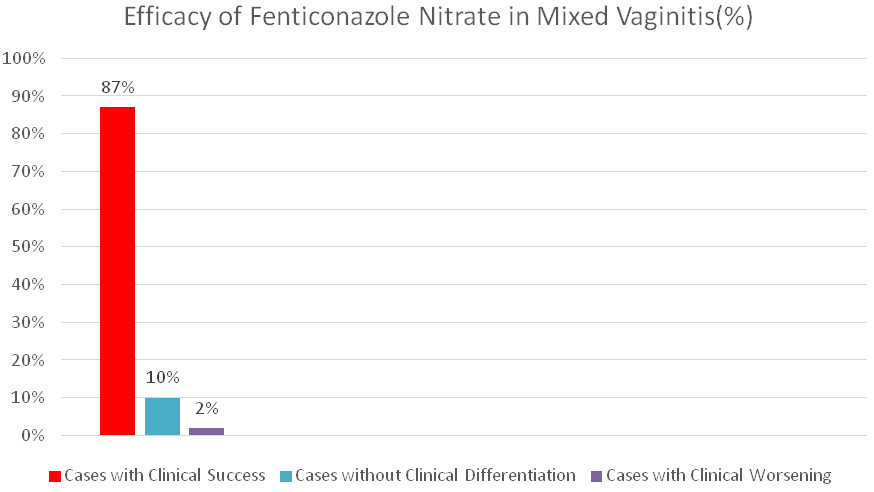
Figure 1 Overall clinical response rates of Fenticonazole Nitrate on day 7 (Adapted from Bukovsky et al38).
In the study performed by Gorlero et al., it was observed that the symptoms decreased significantly after day 4 and the infection improved microscopically in 96 patients with mixed vaginal infections who were administered fenticonazole 600 and 1000 mg vaginal pessaries. Based on these findings, researchers recommend the use of fenticonazole for the treatment of mixed infections. The same research group detailed the researches in 1994 and repeated them on 61 patients with only Trichomonas vaginalis and as a double-center study and reported that the patients recovered completely microscopically and symptomatically with the double dose regimen on day 7 (Table 2).42,43
|
Total Scores |
600 mg Ovules (n:21) |
1000 mg Ovules (n:17) |
Placebo (n:20) |
|
Baseline |
7.85±0.74 |
7.70±0.75 |
7.45±0.51 |
|
Final |
3.47±0.63* |
3.00±0.60* |
5.10±0.87 |
Table 2 The mean values of the symptomatic scores (Adapted from Gorleo et al.43)
*P < 0.01 versus baseline
In a study conducted by Manth et al. in 1989, the efficacy of fenticonazole 600 and 1000 mg vaginal pessaries in Trichomonas vaginalis infection was compared. Treatment success was achieved for both strengths and there was no statistically significant difference in terms of therapeutic efficacy.45
In the study performed by Naud et al., the efficacy of fenticonazole in the treatment of aerobic bacterial vaginitis was evaluated in a pilot, prospective, randomized, double-blind, placebo-controlled study on 47 women. In the study, fenticonazole nitrate 5 g 2% vaginal cream was applied to the vagina for 7 days. The overall cure rate in the fenticonazole group was 85% (p = 0.003). The relapse rate was lower with fenticonazole compared to placebo (11,8% vs 50%). The safety profile of fenticonazole was found to be comparable to placebo.44
In a multicenter, prospective, open-label study conducted by Alba et al., the effect of fenticonazole was evaluated in 101 patients with single or mixed vulvovaginitis infections involving C. albicans and T. vaginalis and/or G. vaginalis administered once on day 1 and 3 as a 1000 mg vaginal ovule. In the evaluation carried out on day 8, the success rate against C. albicans (eradication by microscopic and/or cell culture) was 90%, 70% for T vaginalis (26/29), 67% for G. vaginalis and 45% for mixed infection (all pathogens). After 28 days, no relapse was reported for C. albicans and T. vaginalis, while relapse rates of 27% for G. vaginalis and 23% for mixed infection were reported. Overall, eradication of all disease-causing pathogens was achieved in 67% of total patients. The results of the study show that intravaginal pessaries containing fenticonazole nitrate are an ideal first-line therapy in vulvovaginitis with acceptable broad-spectrum efficacy against the most common pathogens and with low relapse rates.39
In the study of Schneider et al., the therapeutic activity of a single dose of fenticonazole 600 mg ovule was examined in 72 women with only vaginal candidiasis or vaginitis caused by other infective agents other than Trichomonas. Clinical and mycological/microbiological criteria were used for diagnosis and recovery. Symptom scores for erythema, pruritus, discharge, and edema were reduced by more than 90% within 1 week. Clinical improvement was achieved in 69 of 72 patients.31
In addition to these clinical studies, Veraldi and Tumietto, in three systematic literature reviews, emphasized the clinical usefulness of fenticonazole as a first-line agent in the treatment of vulvovaginal candidiasis, mixed, and co-infections.8,11
A number of open, controlled studies on fenticonazole are available in the field of dermatology. In general, clinical evidence shows that different formulations of fenticonazole are effective in the treatment of cutaneous fungal infections, with side effects being rare and mild. Fenticonazole has generally been compared with miconazole, econazole, bifonazole, naphthalene, clotrimazole, ciclopiroxamine, and placebo in these studies. It has been observed that it is mostly used in 2% cream form as well as 2% solution, lotion, spray, foam, gel and powder pharmaceutical forms. Studies have been carried out on tinea pedis, cutaneous mycoses, pityriasis versicolor, dermatophytes, candidiasis, dermatomycoses, superficial mycoses and otomycoses.8
The first study on the efficacy and tolerability of fenticonazole was published in 1984 by Lepine et al. 2% fenticonazole gel was applied twice a day for 28 days to 28 patients with dermatophytosis, and clinical improvement was observed at week.8,46 One year after this study, researchers named Persi and Rebora applied 2% fenticonazole cream to 46 patients with superficial mycosis for 13-28 days and observed clinical and mycological remission in 35 patients.8,47
In 1986, Colerico et al. applied 2% cream, spray, and lotion forms of fenticonazole to 45 patients and obtained good clinical results in all.8,48
In a study in which 2% fenticonazole cream was applied to 30 patients with dermatomycosis and 2% fenticonazole lotion was applied to 10 patients with pityriasiss versicolor twice a day for 5 weeks, it was observed that 28 patients with dermatomycosis and all patients with pityriasiss versicolor were successfully treated.8,49
In 1987, 30 patients with candidiasis, tinea versicolor and epidermomycoses were administered a single daily dose of fenticasole; it was observed that all candidiasis and pityriasis patients responded well to 28-day treatment, and 8 of 10 epidermomycoses patients to 32-day treatment.8,50
In a large, multicenter clinical study in 1988, fenticonazole was administered once or twice daily to 760 patients with superficial mycosis in cream, spray, and powder formulations; the first mycological negativity was observed on days 28-35 of treatment; mycological negativity was reported at week 50 of treatment in 100% of patients with tinea versicolor and 95% of patients with candidiasis. Side effects were detected in 29 patients, and treatment was terminated in only 8 of them.8,50
In another study, in which the cream or lotion form of fenticonazole was applied twice a day for 2 weeks to 51 patients with superficial mycosis and 30 patients with pityriasis versicolor, the clinical and mycological treatment results were evaluated after 4 weeks and it was shown that the absorption of fenticonazole from the application area into the plasma was very.8,49,51,52
It was observed that clinical and mycological total remission was achieved after 2 weeks with fenticonazole treatment applied to 50 patients with otomycosis.8,53
Fenticonazole-miconazole comparative studies
The first double-blind fenticonazole-miconazole comparison study was performed by Stetter in 1984. Both antimycotic agents in 2% cream form were administered to 30 patients with dermatophytosis symmetrical lesions twice a day until improvement was observed within the maximum treatment period (28 days); the fastest improvement in lesions and symptoms was observed in the fenticonazole group.8,54
In a randomized, multicenter double-blind study of 53 patients with dermaophytosis and pityriasis versicolor, both drugs were administered twice daily for 4 weeks; at the end of the treatment, 23 of 25 patients (92%) treated with fenticonazole and 19 of 24 patients treated with miconazole had negative mycological results.8,55
In the study performed by Clerico et al.,8,56 in 40 patients with tinea versicolor, candidiasis or dermatophytosis treated with 2% fenticonazole or miconazole cream; clinical response was observed in 16 of 20 patients treated with fenticonazole, whereas clinical response was observed in 14 of 20 patients treated with miconazole.
Fenticonazole-Clotrimazole comparative studies
In a double-blind study, 2% fenticonazole cream was compared to 1% clotrimazole, both drugs were administered twice daily for 4 weeks. Healing was found in 18 of 21 lesions treated with fenticonazole and in 13 of 21 lesions treated with clotrimazole.8,54
Fenticonazole-Econazole comparative studies
52 patients with dermatomycosis were treated with fenticonazole 2% or econazole 1% twice daily for 4 weeks; clinical and mycological improvement was observed in 15 of 28 patients treated with fenticonazole and only 10 of 24 patients treated with econazole. These findings have been reported that the efficacy of fenticonazole occurs faster than econazole.8,54
In the study conducted by Rabbiossi et al., 2% fenticonazole foam was compared with 1% econazole foam on 40 patients with pityriasis versicolor, and both drugs were applied once a day for 2 weeks. 100% mycological negativity was observed in both groups.8,57
In another study, 2% fenticonazole lotion or 1% lotion was applied to patients with cutaneous mycosis once a day for 2-4 weeks, and 72,7% of the patients in the fenticonazole group and 71% of the patients in the econazole group improved clinically and mycologically.8,58
Fenticonazole-Bifonazole comparative studies
In one study, 2% fenticonazole cream or 1% bifonazole cream was applied to 41 patients with dermatomycosis once a day for 4 weeks(66), and in another study conducted in 46 patients with pityriasis versicolor, 2% fenticonazole lotion or 1% bifonazole lotion was applied once a day for 3 weeks, and a faster therapeutic activity was observed with fenticonazole compared to bifonazole.8,59
Fenticonazole-Ketoconazole comparative studies
In an in vitro activity study against pathogens isolated from 51 patients with superficial skin mycosis, fenticonazole was observed to be as effective as ketoconazole, miconazole, and itraconazole;50 and in a clinical study conducted in 81 patients with chronic candidiasis, it was observed that it was as effective as ketoconazole and nystatin in oral lesions.8,60
Fenticonazole-Naphtidine comparative studies
A multicenter, double-blind study was performed on 100 patients with cutaneous fungal infections, comparing the efficacy and tolerability of 2% fenticonazole and 1% naphtidine spray formulations. At the beginning of the study, 33,3% of the patients in the fenticonazole group and 20,8% of the patients in the naphtidine group had found to have Candida albicans infection, however at the end of the treatment, only 6,3% of the patients in the fenticonazole group and 10,4% of the patients in the naphtidine group were positive. When symptoms were evaluated, significant improvement (90%) was observed in patients in both groups. In the follow-up studies, mycological recurrence was found in only 1 patient (3,3%) in the fenticonazole group and in 2 patients (6,3%) in the naphtidine group among the patients who recovered completely at the end of the treatment.8,61
Comparative studies with fenticonazole-cycloproxolamine
In a double-blind study of 100 patients comparing fenticonazole spray with 1% cycloproxolamine, both antimycotics were administered once daily for 2-4 weeks; clinical improvement was found to be 91,8% with fenticonazole and 89,8% with cycloproxolamine.8,62
Vaginal infection or vaginitis is an infection that occurs due to pathogenic microorganisms found in the normal vaginal flora or transmitted during sexual contact.63 In vaginitis, which also means inflammation of the vaginal mucosa, the main problem is the dysfunction of the vaginal flora.64,65 Unprotected sexual intercourse, multiple sexual partners, vaginal douching, long-term antibiotic use, diabetes and smoking habits can be counted among the risk factors for the development of vaginal infections.66 Common causes of vaginal infections in the world are bacterial vaginosis, vulvovaginal candidiasis and trichomoniasis. Bacterial vaginosis accounts for approximately 30% of vaginal infections.67 Although the incidence of candida, which is another common vaginal infection factor, is not known exactly, it is estimated that 75% of women can be infected with candida species at any time in their lives.68 While Candida Albicans is the most common pathogen detected in vulvovaginal candidiasis,69 Gardnerella Vaginalis is the most frequently detected pathogen in bacterial vaginosis. Co-infection with candida can be seen in approximately 30% of bacterial vulvovaginitis.19
Vaginitis are a widespread condition associated with a number of bothersome symptoms, either subjective (e.g., pain, burning and pruritus) or objective (e.g., stream, redness and swelling). One-third of women experience vaginitis at some point in their lives Mixed vaginitis is vaginitis with at least two of the bacterial and/or fungal factors. Mixed infections can be controlled with antifungal, antibacterial and antiprotozoal combined treatments.19 This combined treatment may cause side effects and an increased risk of resistance. Therefore, multi-acting drugs such as fenticonazole are valuable in the treatment of vaginitis. In addition, considering the physical properties such as the size, shape and consistency of the vaginal passeries to be preferred in the treatment planning of vaginitis should be taken into account, as these are factors that can significantly affect the success of the treatment.68
The clinical efficacy of topical fenticonazole in vaginal infections caused by Candida, Gardenella and Trichomonas has been evaluated in 17 randomized controlled studies involving more than 1000 patients.11,19
Fenticonazole nitrate is an imidazole derivative with a broad spectrum of antimycotic activity against dermatophytes and yeasts in vitro and clinical studies. Fenticonazole also shows antibacterial activity and high cure rates and low relapse rates against bacteria commonly associated with superficial fungal and vaginal infections. In addition, its anti-parasitic effects against Trichomonas vaginalis have also been demonstrated in in vitro and clinical studies.11,14,19
While fenticonazole provides a cure rate of 85% in vaginitis of bacterial origin, the cure rate is 97,5% in vaginitis caused by Candida albicans.41 In vaginitis caused by Trichomonas vaginalis, a cure rate of up to 80% is observed with the use of topical fenticonazole.43 Studies conducted with fenticonazole have shown that up to 90% of symptoms such as pruritus, burning, redness, discharge and edema, which are frequently observed in vaginitis, can be improved.19
Conditions such as hormonal changes in the vaginal epithelium, increase in vaginal pH from 3.5-4.5 to 6-8, urinary and/or fecal incontinences, atrophy in the vaginal tissue, trauma in the prepubertal, pubertal or postmenopausal periods cause the vagina to become open to infection and as a result, vaginitis develops by causing the lactobacilli in the flora to leave their places to the mixed flora, predominantly to pathogenic cocci.2
Improper treatment of vaginal infections can affect the ecological characteristics of pathogenic fungi and worsen the course of infections. In vaginal infections, systemic (oral) and multi-agent treatments can also be used as first-line therapy. However, an effective topical treatment, when used instead of systemic drugs, provides protection in terms of intestinal flora and systemic side effects, while reducing the risk of bacterial resistance development. Topical fenticonazole treatment of vaginitis is a treatment option that does not contain risks such as microbial resistance development and deterioration in intestinal microflora due to systemic treatments.
It has been reported that recurrent vaginal infections can be prevented by preserving the vaginal lactobacillus flora. In this respect, fenticonazole differs in that it has high MID rates against the three main lactobacillus strains called Lactobacillus gasseri, Lactobacillus jensenii and Lactobacillus crispatus, and lower efficacy on the vaginal flora compared to miconazole and metronidazole.70
In vitro time kill data demonstrated the supra-MIC potential microbicidal activity of fenticonazole. These concentrations have been reported to be readily attainable in topical therapy with fenticonazole.16 Data suggest that applied fenticonazole concentrations can also be effective against Candida albicans and Staphylococcus aureus or other bacterial strains present in mixed vaginal infections, thereby reducing the risk of developing resistance.
Fenticonazole's broad spectrum of action against fungi, bacteria and antimicrobial activity against mixed infections, its safe systemic side-effect profile, and ease of administration allow it to be applied as a first-line treatment option in mixed vaginitis without the need for a test to identify the pathogen.19
Fenticonazole has a broad spectrum of antimicrobial activity with its antifungal, antibacterial and antiparasidic effects. Therefore, fenticonazole is an ideal treatment option for the topical treatment of mixed vaginitis involving mycotic, bacterial, dermatophytes and/or Trichomonas spp. Intravaginal administration of fenticonazole has a rapid onset of antimicrobial action, and clinical efficacy is usually observed within the first days of treatment initiation. Topical fenticonazole is very well tolerated; adverse effects are usually mild and transient. Fenticonazole has a broad safety profile and does not exhibit systemic activity.19 Given the increasing incidence of fungal, bacterial and mixed vaginal infections, fenticonazole is an important member of the topical antimycotic agents.
In the light of studies where it was reported that no test used in the diagnosis of vulvovaginitis may be sufficient to detect the causative agent, in a study carried out with the participation of 300 women in Turkey, it is also reported that fenticonazole is a topical agent that can be preferred in the first-line treatment of mixed-type vaginitis without the need for testing to identify the pathogen.71 In studies where fenticonazole cream and ovule forms are used together, higher clinical success and also lower recurrence are observed compared to ovule or cream treatments alone.29
Fenticonazole has a broad spectrum of antimicrobial activity with its antifungal, antibacterial and antiparasidic effects. It is an ideal topical alternative with its high clinical efficacy in Candidal vulvovaginitis caused by Candida albicans, bacterial vaginosis caused by Gardnerella vaginalis, gram (+) and/or gram (-) bacteria, and Trichomonal vaginitis caused by Trichomonas vaginalis and mixed vaginitis caused by these pathogens and its excellent tolerability. Fenticonazole has lower efficacy against the three main lactobacillus strains called Lactobacillus gasseri, Lactobacillus jensenii and Lactobacillus crispatus found on the vaginal flora compared to miconazole and metronidazole with high MID rates. Fenticonazole is a treatment option with efficacy, safety and excellent tolerability that can be the first choice empirically in the first-line treatment of mixed-type vaginitis, which has been shown to be effective in fungal, bacterial and mixed-type vaginal infections with its broad spectrum of action in various clinical studies.
None.
Gokhan Faikoglu, Kubra Saygisever-Faikoglu and Fatmanur Otmar Ozcan are employees of Recordati.

©2022 Saygisever-Faikoglu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.