eISSN: 2379-6367


Research Article Volume 8 Issue 4
1Department of Neuropharmacology, Institute of Experimental Medicine, Russian Academy of Sciences, Russia
2Department of Biochemistry, Institute of Experimental Medicine, Russian Academy of Sciences, Russia
Correspondence: Irina V Okunevich, PhD (Pharmacology), Senior Researcher, SV Anichkov Department of Neuropharmacology, FSBSI, Institute of Experimental Medicine, 12 Acad. Pavlov St., 197376 St. Petersburg, Russia, Tel (812)2345447
Received: June 28, 2020 | Published: July 20, 2020
Citation: Okunevich IV, Klyueva NN, Parfenova NS, et al. The corrective action of original cholesterol oxidase preparation: in vitro and in vivo use. Pharm Pharmacol Int J. 2020;8(4):209-213. DOI: 10.15406/ppij.2020.08.00298
Atherogenic dyslipoproteinemia (DLP) is one of the modifiable risk factors of atherosclerosis (AS). For the primary prevention of DLP and reduce the risk of progression of the AS, it is necessary to use low cholesterol (Ch) foods and apply modern effective lipid-lowering agents that improve the blood lipid profile. Relevant is the search for various biologically active compounds that correct of lipids in DLP. The original preparation of cholesterol oxidase enzyme (PChO) of microbial origin was the subject of this study. In vitro experiments evaluated the ability of PChO to actively bind cholesterol in food. To assess the effect of PChO under conditions of moderate nutritional DLP, in vivo experiments were carried out in three species of animals (rats, guinea pigs, rabbits). It is established that when modeling DLP, the studied agent PChO when administered orally has a pronounced hypolipidemic effect. It is noted that under these conditions, after applying PChO, the disturbed blood lipid spectrum is significantly improved. When analyzing the results of ultracentrifugation of the blood serum of experimental animals, a decrease in the content of atherogenic (LP)–VLDL, LDL, and a positive increase in the concentration of antiatherogenic HDL, capable of accepting Ch were detected.
Keywords: atherosclerosis, dyslipoproteinemia, cholesterol, cholesterol oxidase
Atherosclerosis (AS) is a multifactorial, chronic, progressive cardiovascular disease that affects elastic arteries, which is characterized by the deposition of lipoproteins (LP) and cholesterol (Ch) pathologically delivered by them in the intima of vessels.The consequence of this is the formation of atherosclerotic plaques, narrowing the lumen of the arteries, which leads to dangerous ischemia and fibrotic changes in the organs. AS-a socially significant, costly disease that requires adequate treatment to reduce disability and mortality of people.1 Atherogenic dyslipoproteinemia (DLP) is one of the modifiable risk factors for AS. Synthetic lipid-lowering drugs are used for the correction of DLP, which, in addition to their specific activity, have side reactions. Relevant is the search for biologically active agents of different origin, correcting the disturbed lipid metabolism in DLP. One of the ways of primary prevention of DLP is to limit the consumption of Ch from food. For this purpose use lipid reducing agents that prevent the absorption of excess Ch in the intestine (drug cholestyramine in doses of 18-24 g per day). In addition, patients with AS are advised to eat foods that are poor in Ch. The choice of food with a low content of Ch is not an easy task, because the patient must exclude milk, eggs, meat and butter from his diet. We have suggested that for obtaining products with a reduced content of Ch, it is advisable to use natural enzyme preparations obtained by biotechnology. Our attention was attracted by the original domestic preparation of the cholesterol oxidaseenzyme (PChO). It is known that the source of the enzyme ChO [EC 1.1.3.6.] is Streptomiceslavendulae bacteria. ChO belongs to the class of oxido-reductases, has a molecular mass of 55 kDa and catalyzes redox reactions, in particular, oxidation of the hydroxyl radical in the C3 position to form ketone (cholest-4-en-3-one) and hydrogen peroxide. On the initiative of academician A.N. Klimov, a well-known specialist in the field of DLP and AS studies, a sample of preparation of ChO (PChO) was transferred to the lipid metabolism laboratory of the Department of Biochemistry of the Institute of Experimental Medicine (St. Petersburg, Russia) at primary research to detect biological activity and antiatherosclerotic action.
In Part I of this study, it is planned to determine in vitro the binding ability of PChO, and in Part II in vivo experiments on models of alimentary DLP in experimental animals to reveal its hypolipidemic activity.2,3
Drugs
The technology of obtaining PChO from Streptomiceslavendulae strain VKMA-5921 of soil origin was developed at the Institute of Antibiotics and Medical Enzymes (St. Petersburg). Materials of method for obtaining PChO closed of the institution and are patently capable.
In part I of the study
In vitro experiments, we used the method of reducing cholesterol content in food products (milk, eggs) to assess the binding capacity of PChO. First, the content of Ch in the products was determined, and then the studied agent, PChO was added to it in an amount sufficient to reduce Ch. It has been suggested that reduction ofCh content in food products by 1.5-2 times will contribute to a decrease in Ch level in blood serum.
In part II of the study
In vivo experiments were carried out in 3 three species of animals (rats, guinea pigs, rabbits) to determine the effect of PChO under conditions of moderate nutritional DLP.
Animals
In preliminary in vivo chronic experiments in 4 species of animals–males (75 mice, 150 rats, 14 rabbits, 10 dogs), low toxicity, good tolerability, effective doses and pronounced anti-atherosclerotic effect of PChO were established. The experiments were conducted in adult animals-males: 120 rats (200-300g), 30 guinea pigs (350-380g) and 18 rabbits (3.0-3.5kg).Our experimental studies were carried out in accordance with the Rules of work on laboratory animals kept in standard vivarium conditions, with free access to water and food.
Methods
Experimental nutritional DLP was created based on the instructions of the chapter on the preclinical study of lipid-lowering and anti-atherosclerotic drugs of the Russian Methodological Recommendations (2012). One of the authors of the article, Senior Reseacher IV Okunevich took part in the development of experimental nutritional models of DLP.4 In rats, alimentary DLP was createdfor 20 and 30 days by feeding an atherogenic diet in the form of a hypercholesterinemic diet (HCh diet) enriched with dietary Ch. The HCh diet contained a very heated mixture of 5% cholesterol in sunflower oil and 0.12% methyl thiouracil (MTU), an essential thyroid suppressor agent. DLP in guinea pigs was created for 20 days by feeding HChdiet with a containing 0.5 g/kg Ch and a very heated mixture of fats (sunflower oil/lard fat 3:1). DLP in rabbits was modeled for 12 and 24 days using 5%food Ch mixed with 100 g of fresh cabbage. After 18 hours of starvation, rats and guinea pigs were killed by rapid decapitation, rabbits - by air embolism. The obtained serum was determined by the content of total Ch, triglycerides (TG), Ch of high-density lipoproteins (HDL-Ch) using reagent kits “Ranbaxy” (England). According to the standard formula A.N. Klimov was calculated atherogenic index of blood (IA) - [total Ch–HDL-Ch/HDL-Ch]. The blood serum of experimental animals was investigated using the method of ultra-centrifugation in the density gradient KBr for analyzing the distribution of the spectrum of lipoproteins (LP) - intermediate density LP (IDL), very low-density LP (VLDL), LP of low-density LP (LDL) and high-density LP (HDL). In the liver of rats and guinea pigs, the total Ch and TG content was determined using specific color reactions after extraction with isopropanol.
Statistical analysis
Statistical data processing was performed using with one-way analysis ANOVA test, at p<0.05, then post hoc comparison were made.
Results of Part I–the in vitro experiments
Study I: №1 showed the binding of cholesterol in milk. Before starting the experiment in raw milk containing 6% fat, the concentration of total Ch was determined. In the test milk the initial concentration of Ch was 0.42 mg/ml. Then 400 units of PChO (initial activity 16 units/ml, in 0.001 M sodium phosphate buffer, pH 7.4 at the rate of 0.4-0.5 units of enzyme per 1 ml of milk) were added to 1 L of milk. The resulting milk mixture was stirred for 20 seconds and incubated at room temperature for 4 hours. The final concentration of Ch in the test milk was 0.22 mg/ml. Thus, in these experimental conditions, we found a decrease in Ch content in milk by 48%. In preliminary experiments, it was noted that after 1 hour of incubation, the concentration of Ch decreased by 25% in milk. However, our research has shown that it is not advisable to incubate the mixture for more than 5 hours, or to raise the reaction temperature. This affects the quality of milk and does not lead to a proportional increase in the amount of oxidized Ch in this product.
Study I: №2 showed the binding of cholesterol in egg yolks. In the experiments with eggs, in contrast to the experiments with milk, the initial concentration of PChO was increased 10 times. This is explained by the well-known fact that eggs are the richest in Chl (each yolk contains about 300 mg of Ch). The sample was prepared from 5 eggs. The yolks were separated from the proteins and the initial amount of Ch was determined in 75 ml of yolks. The initial content of Ch was 15.64 mg/ml. Added 4000 units PChO (activity 16 units/ml) in 0.001 M sodium phosphate buffer, pH 7.4 (4-5 units per ml of yolk). Next, the mixture of egg yolks with PChO was actively stirred for 20 seconds, incubated in a thermostat for 4 hours at 37°C with constant stirring. The final Ch content in 5 egg yolks was 8.06 mg/ml, i.e. the Ch content decreased by 51%.Thus, in experiments in vitro, the pronounced binding capacity of the original PChO was shown.
Part II: The results of the in vivo experiments
In our preliminary experiments, acute toxicity and LD50 (doses letalis50) of PChO in 75mice and 75 rats could not be determined; administration of large doses of 500 units and 1000 units PChO did not cause the death of animals. In test experiments, it was found that oral administration of PChO at doses of 300-400 units of animals tolerated without complications. In chronic experiments on 75 rats and 10 dogs, it was established that daily administration of an oral dose of PChO (with an activityof0.16 units/mg to 1.0–20.0 units/mg) does not have a toxic effect in the organism of rats (3 months) and dogs (5 months). Analysis of the effect of PChO on vital organs and systems, the activity of the gastrointestinal tract, blood by the form elements and the morphology of the organs (heart, liver, kidneys, adrenal glands, spleen) did not reveal any pathological changes in the experimental animals. Thus, in preliminary experiments it was shown that the object of study of PChO is of low toxicity.
The results of study experiments of the effect of PChO in the development of moderate alimentary DLP are presented in Tables 1-4. As can be seen from Table 1, after the drug oral administration of PChO to guinea pigs contributed to a decrease in the level of total Ch and TG in the serum and in the liver of these animals. Thus, Ch level in the blood decreased by 49%, in the liver - by 26%, TG content in the blood serum decreased by 21%, in the liver-by 25%.The experiment indicated a pronounced hypolipidemic effect of the studied agent PChO on the DLP model in guinea pigs.
The groups of animals |
Blood serum total |
Blood serum |
Liver total |
Liver triglycerides (mg/g) |
1. Normal guinea pigs |
1,19±0,13 |
0,61±0,14 |
2,5±0,07 |
5,8±0,4 |
2.HCh diet |
6,49±0,72* |
18,0±0,36* |
13,8±0,09* |
26,5±3,2* |
3. HCh diet + PChO |
4,14±0,54# |
14,3±0,27# |
10,5±0,08# |
20,0±1,7# |
Table 1 Effect of the enzyme cholesterol oxidase (the preparation PChO) at a dose of 20 units orally for lipid changes in serum and liver of guinea pigs fed the HCh diet for 20 days
Note: *-the differences are significant in comparison of groups 1 and 2 at p <0.05; #-the differences are significant in comparing groups 2 and 3, with p<0.05. In each group were of 10 animals.
The groups of animals |
n |
Total Ch of blood serum (mM/l) |
TG of blood serum (mM/l) |
IA (units) |
||||
Initial data |
After 12 days |
After 24 days |
Initial data |
After 12 days |
After 24 days |
12 days |
||
1. Norma |
5 |
1,1±0,21 |
1,2±0,44 |
1,4±0,10 |
0,64±0,10 |
0,71±0,28 |
0,68±0,09 |
1,0 |
2. HCh diet |
5 |
1,2±0,16 |
29,7±3,80* |
45,0±2,82* |
0,62±0,11* |
1,67±0,32* |
2,45±0,24* |
58,4 149,0 |
3. HCh diet + PChO |
8 |
1,2±0,05 |
24,5±2,70*# |
11,7±2,64*# |
0,61±0,15*# |
0,97±0,11*# |
0,42±0,21*# |
48,0 38,0 |
Table 2 Effect of the preparation of enzyme cholesterol oxidase (PChO) at a dose of 20 units orally for changes in serum lipid levels in rabbits fed the HCh diet during 12 and 24 days
Note: n-the number of animals in the group, *-the differences are significant in the comparison of groups 1 and 2, * #-the differences are significant in the comparison of groups 2 and 3, with p<0.05; n-the number of animals in each group.
The groups of animals |
Ch of blood serum (mM/l) |
TG of blood serum (mM/l) |
HDL-Chof blood serum (mM/l) |
Index of atherogenity (IA) |
1. Normal rats |
1,40±0,03 |
0,78±0,02 |
0,93±0,07 |
0,50 |
2. HCh diet |
4,69±0,13* |
0,42±0,05* |
0,55±0,06* |
7,43 |
3.HCh diet+PChO 2 units |
4,01±0,15*# |
0,56±0,07* |
0,64±0,08*# |
5,26 |
4.HCh diet+PChO 4 units |
3,96±0,17*# |
0,52±0,04* |
0,73±0,06*# |
4,43 |
5.HCh diet+PChO 8 units |
3,78±0,31*# |
0,50±0,03* |
0,81±0,09*# |
3,66 |
6. HCh diet+ PChO 10units |
2,92±0,18*# |
0,51±0,08* |
0,96±0,08*# |
2,04 |
7.HCh diet+PChO 20 units |
2,79±0,27*# |
0,49±0,06* |
0,97±0,05*# |
1,74 |
Table 3 Effect of different doses of the preparation of the enzyme cholesterol oxidase (PChO) on lipids in rat blood serum when administered orally during 30 days
Note: *-the differences are significant in comparison of groups 1 and 2 at p <0.05; #-the differences are significant in comparison of group 2 (HCh diet) and 3, 4, 5, 6, 7 groups, with p <0.05. In each group were of 12 animals.
The groups of animals |
Total Ch of blood |
TG of blood |
Liver total Ch (mg/g) |
Liver TG (mg/g) |
1. Normal rats |
1,32±0,08 |
0,73±0,06 |
4,45±0,38 |
7,53±0,91 |
2.HCh diet |
3,17±0,13* |
0,42±0,05* |
21,15±0,96* |
23,4±1,03* |
3. HCh diet+ PChO 20 ед. |
1,71±0,05*# |
0,32±0,07* |
14,48±1,03*# |
12,0±1,13*# |
Table 4 Effect of the preparation of the enzyme cholesterol oxidase (PChO) at a dose of 20 units orally on serum and liver lipids of rats fed the HCh diet during 20 days
Note: *-the differences are significant in comparison of groups 1 and 2 at p <0.05; #-the differences are significant in comparing groups 2 and 3, with p<0.05. In each group were of 12 animals.
Table 2 shows the results obtained after oral administration of PChO and HCh diet to rabbits. From table 2 it can be seen that after 12 days and 24 days of feeding animals with HCh diet, the content of Ch and TG in blood serum significantly increased (group 2). The oral administration of the PChO+HCh diet together to rabbits for 12 days contributed to a significant decrease in the level of total Ch by 44%, and within 24 days by 58%. The level of TG in the serum after 12 days decreased by 32% and by 73% after 24 days (Table 2, group 3). When analyzing the degree of blood atherogenicity in rabbits treated with PChO, after 24 days, a decrease in the calculated IA compared with the value of IA in the group of untreated rabbits was noted (Table 2, groups 3 and 2). The data obtained on the DLP model in rabbits testified to a pronounced hypolipidemic effect of PChO.
Table 3 presents the results of experiments on the DLP model in rats. As can be seen from table 3, with the administration orally of different doses of PChO, it was found a significant decrease in the serum total Chfrom 15% to 59% was observed (groups 3-7, compared with group 2). A positive increase (to normal) in Ch concentration of antiatherogenic HDL compared with a group of rats in comparison in group without treatment was also noted. When analyzing the degree of serum atherogenicity, a decrease in the value of estimated IA from 5.26 to 1.74 is shown (Table 3).
Table 4 presents the results of DLP modeling in rats for 30 days. In the group of rats fed the HCh diet and PChO, a decrease in serum total Ch level by 47% and in the liver by 34% was shown. The lipid reducing effect of PChO was noted in a significant decrease in the liver TG content by 49% (Table 4, groups 3 and 2). Thus, in the rat model of alimentary DLP, the hypolipidemic effect of the studied agent PChO was detected.
The figure 1 shows photographs of serum samples of guinea pigs, rabbits and rats obtained after ultracentrifugation by the method of separation of the LP in the density gradient KBr. It can be seen from the figure that the HCh diet causes a violation of the serum LP profile in all animals. This increases the layer corresponding to the content of atherogenic LP–IDL, VLDL, and LDL, and decreases the layer of antiatherogenic HDL. However, the long-term administration of the PChO in the condition DLP (HCh diet) contributes to the improvement of the blood profile of the LP: there is an enlightenment of the layer of corresponding to atherogenic LP and an increase in the size of the layer corresponding to antiatherogenic HDL is observed.
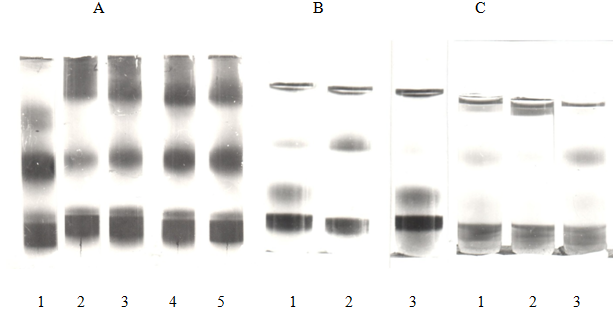
Figure 1 Corrective effect of preparation cholesterol oxidase (PChO) in the lipoproteins (LP). Photographs of serum samples of rabbits A (1-5), rats B (1-3), guinea pigs C (1-3) obtained after ultracentrifugation by the method of separation of the lipoproteins (LP) in the density gradient KBr (normal and after feeding HCh diet and HChdiet+PChO)
Note: Sample 1–norma-intact animals: rabbits–A, rats-B, guinea pigs-C
Sample 2-hypercholesterol diet (HCh diet) in A, B, C
Sample 3–HChdiet+the preparation of cholesterol oxidase (PChO, 20 units) in B, C
Different doses of PChO: sample 3(4 units), sample 4 (10 units), sample 5 (20 units) in A
It is known that enzymes are highly active, and most importantly, non-toxic catalysts with exceptional substrate specificity, without which many vital biochemical processes are impossible in the body. Modern biotechnological use of preparations of the enzyme ChO, obtained from various sources, its chemical and physiological properties are of great interest to researchers abroad.3,4 In Russia, the ChO enzyme itself is used in laboratory diagnostics to determine the level of total Ch and HDL-Ch in serum. Created original means based on ChO-active agent-PChO from Streptomiceslavendulae strain VKMA-5921 of soil origin is studied for the first time. In preliminary experiments, it was found that PChO is a low toxic agent. In mice, rats, rabbits and dogs, low acute and chronic toxicity was found, as well as good tolerability of the PChO test agent. In rabbits with experimental AS it was shown the significant lowering effect of PChO in the content of blood serum Ch. When analyzing the degree of development of an experimental AS, the percentage of atherosclerotic aorta lesions in the experimental group of 7 rabbits fed the PChO + HCh diet was 28% against 67% in the control group of 7 animals (HCh diet) without treatment. In experiments on the model of alimentary AS in rabbits, the significant anti-atherosclerotic effect of PChO was detected. Thus, these results served as the basis for continuing the study of the properties of the cholesterol oxidase preparation-PChO.
In the present study, the binding capacity of PChO in food was determined. In the experiments performed in vitro, the PChO property was found to reduce the content of Ch in raw milk and egg yolks by a factor of 1.5 to 2. In experiments in vivo, we found that PChO has a pronounced hypolipidemic effect in the moderate nutritional DLP in rats, guinea pigs and rabbits. The use in experiments of 3 types of experimental animals - rodents is dictated by the peculiarities of their blood lipid spectrum. In rabbits, the main amount of Ch is distributed evenly among the fractions of atherogenic LP (VLDL and LDL) and high-density anti-atherogenic LP (HDL). In rats, the main pool of Ch is in antiatherogenic HDL, and in guinea pigs - in atherogenic fractions of VLDL and LDL. Guinea pigs are more sensitive to the creation of stimulated DLP than rabbits, and especially rats. Rats are less sensitive than rabbits to modeling alimentary DLP. Therefore, it is necessary to introduce a special HCh diet for a long time. HCh diet should contain not only an excess of Ch, but also high doses of such damaging agents as the thyroid suppressor substance methyl thiouracil (MTU), vitamin D2, sodium cholate. Using MTU, the excess content of food Ch and Ch in a very heated mixture of vegetable and animal fat, we managed to create a moderate DLP in rats, rabbits, guinea pigs, and to identify the lipid-lowering effect of PChO.
In in vitro experiments, the ability of PChO to actively bind cholesterol in food products was determined. These data obtained can be useful for creating dietary products, poor Ch. In in vivo experiments on 3 types of animals-rodents under conditions of moderate DLP, the hypolipidemic effect of the preparation of the enzyme cholesterol oxidase (PChO) was established. The results demonstrated that PChO did not cause any abnormalities in the state and behavior of experimental animals. Our findings had perspective of further development and implementation of this original preparation. In connection with the results obtained, we consider it promising to continue the study of the biological activity and mechanism of action of this original remedy containing enzyme – cholesterol oxidase (ChO) for effective pharmacology and future use in medicine.
Animal experiments were carried out in compliance with humanity (European Community Directive No. 86/609) and approved by the Ethics Committee of the Institute of Experimental Medicine.
None.
Authors declare that there is no conflict of interest.

©2020 Okunevich, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.