eISSN: 2379-6367


Research Article Volume 5 Issue 4
School of Studies in Chemistry, Jiwaji University, India
Correspondence: Sami Ullah Bhat, School of Studies in Chemistry, Jiwaji University, Gwalior, India
Received: July 25, 2017 | Published: September 12, 2017
Citation: Bhat SU, Naikoo RA, Tomar R, et al. Synthesis of n-formylation of amines using various ion exchanged forms of zeolite-a as catalysts. Pharm Pharmacol Int J. 2017;5(4):151-157. DOI: 10.15406/ppij.2017.05.00130
A simple, convenient and new efficient procedure was utilized for the synthesis of N-Formylation of amines using Zeolite A as a heterogeneous solid acid catalyst. The main advantage of this protocol is less reaction time, high percentage yield, cleaner and easy work up. The synthesized zeolites were characterized by various techniques Viz, Fourier-transform infrared spectroscopy(FTIR), X-ray diffraction(XRD) and Scanning electron microscopy(SEM). The synthesized products were characterized by HNMR and Fourier-transform infrared spectroscopy(FTIR) techniques. Furthermore the catalyst can be recovered conveniently and reused efficiently.
Keywords: n-formylation, zeolite a, amines, less reaction time
Formamides represent an important class of intermediates in various organic syntheses. They have been used in the synthesis of some pharmaceutically important compounds such as Fluoroquinolones,1 1, 2 dihydro quinolones2 and Nitrogen based heterocyclic compounds.3 In Lewis bases, its main importance is to catalyze some important reactions such as allylation4 and hydrosilyation of carbonyl compounds5 Formamides Act as useful reagent in Vilsmeier Formylation reaction.6 The main application of Formamides is to use in the synthesis of Formamidines7 and in peptide synthesis acts as useful amino protecting group.8 Various methods have been previously reported in literature for the synthesis of N-Formylation of amines and various catalysts were used for this reaction, such as acetic formic anhydride,9 activated formic acid using Dicyclohexylcarbodiimide10 or,1-Ethyl- 3-(3-dimethylaminopropyl) carbodiimide,11 chloral,12 activated formic esters,13 ammonium formate,14 2,2,2-trifluoroethyl formatem,15 ZnO,16 PEG-400,17 Formic acid-ZnCl2,18 polyvinyl alcohol,19 Na+-montmorillonite,20 Nano Cerium Oxide,21 Natrolite zeolite,22 HCOOH Sodium formate,17 Silica-bonded N-Propyl Sulfamic acid,23 TiCl3(OTF),24 CDMT,25 KF-AL2O3,26 Formic acid in Toluene,18 Formic acid in polyethylene glycol,17 Natural HEU Zeolite.27 However above listed methods have some limitations such as long reaction time, formation of undesirable byproducts, hygroscopicity expensive reagents, and thermal instability of the reagents, and high temperature for reaction completion. Keeping in view the above disadvantages, we report a less time consuming method for the synthesis of N-Formylation of amines using various ion exchanged forms of Zeolite A as reusable catalysts.
Preparation of zeolite-A and its various ion exchange forms
For preparing Zeolite A, 0.723 g of sodium hydroxide was dissolved in 80 mL distilled water which was then divided into two equal volumes in polypropylene bottles. To first half, 8.258 g of sodium aluminate was added and mixed gently in capped polypropylene bottle up to its clearance. To the second half, 15.4 g of sodium metasilicate was added and mixed gently in capped polypropylene bottle up to its clearance, thick gel was formed quickly after pouring silicate solution into aluminate solution and polypropylene bottle was kept for 4 h in oven at 99oC. The required mixture was washed by distilled water and centrifuged up to PH ≤ 9 and dried at 100oC .The sample was ground into powder and calcined at 500 oC for 4 h in order to remove water and organic precursors.
Na- form of Zeolite A was converted into H form by the following procedure: we took 9.0 g of Zeolite A, 7.23 g of NH4Cl and 13.80 mL of distilled water and these three components were then mixed with 0.1M hydrochloric acid solution so as to maintain the pH 4 of the solution. The mixture was stirred at 60oC for 6 h. After that the required material was filtered and washed 2-3 times with distilled water. After filtration and washing with distilled water chlorides were removed then NH4-zeolite was placed in an oven at 60oC for 24 h. Finally the ammonium form of Zeolite-A was converted into the H form by calcination over 60 min at 500oC.
Na- form of calcinated Zeolite A was converted into various ion exchanged metal forms by exchanging Na+ ion present on the parent zeolite with other alkali metal ions such as K+, Mg+ by stirring zeolite material in alkali metal solutions. A mixture containing 5 g of Zeolite-A and 144 mL of 0.0125 M metal nitrate (MgNO3, KNO3) was then stirred at 80oC for 24 h. Then the material was recovered by filtration and washed 2-3 times with distilled water. Finally the required sample was dried for 12 h at 120 oC and calcined at 450 oC for 4 h (Figure 1).
Synthesis for the N-Formylation of amines
A mixture of 4-Chloroaniline (1.0 mmol), aq. formic acid (1.2 mmol) and Zeolite A (0.05 g) in a 25 ml conical flask was stirred under solvent free conditions at room temperature for appropriate time (as shown in Figure 1). After reaction was completed (monitored by TLC), ethyl acetate was added and the catalyst was removed by filtration. After removal of solvent, the product was further recrystallized with suitable solvent such as (ether or chloroform). The product structure was confirmed by HNMR, FTIR and compared with samples already obtained by reported methods (Figure 2).
X-ray diffraction
From powder X-ray diffraction (XRD) studies, the catalyst was analyzed by using Shimadzu XRD 6000 model equipment. The operational detailed technique were set as follows: Copper Kα radiation at 40 kV/30 mA, with a goniometer speed of 2°/min and a step of 0.02° in the 2θ range scanning from 10° to 70° for Na-zeolite A and 2θ range scanning from 0° to 80° H-Zeolite A, K-Zeolite A and Mg-Zeolite A.
Scanning electron microscopy
SEM images of calcinated zeolite A, H-form and metal ion exchanged form were obtained by using SEM instrument. SEM images of these materials were taken at 10,000 x magnifications to show their surface morphology and to obtain the clear view of crystals.
Fourier transform-infrared spectroscopy (FT-IR)
For FT-IR analysis, calcined zeolite A, H-form, metal ion exchanged form and reaction products. These samples were subjected to physical treatment with the KBr method, which contains 0.007 g of the sample mixed with 0.1g KBr, grinding and pressing the solid mixture to 5 tons for 30 s to form a pellet then allows the passage of light by using spectrophotometer Shimadzu FT-IR with the wavelength range from 500 to 400 cm−1..
Nuclear magnetic resonance (NMR) spectroscopy.
HNMR spectra were obtained on Bruker 400 MHz spectrophotometer with CDCl3 as solvent using tetramethylsilane (TMS) as an internal standard; the chemical shift values are in 𝛿.
Spectral data of products
N-(4-Chlorophenyl) foramide: MP; 100-103oC; Colour; Creamy white; FT-IR (KBr); 3251, 3050, 1688, 1485, 1398 cm-1, HNMR (δ, 400MHZ, CDCl3): 7.03-7.49 (m-4H, Ar-H), 8.65 (s, 1H-NH), 8.35 (s, 1H-CHO) (Table 1, entry 2 fig 6,7)
N-(3-Nitrophenyl) foramide: MP; 130-132oC; Colour; Creamy white; FT-IR (KBr); 3251, 3072, 1620, 1515, 1340 cm-1, HNMR (δ, 400MHZ, CDCl3): 8.82(s, 1H-NH), 8.79(s, 1H-CHO), 8.22(d, 2H, Ar-H), 7.99(d, 2H, Ar-H) (Table 1, entry 3 fig 8,9)
N-(3, 4-Dichlorophenyl) foramide: MP; 115-120oC; Colour; Creamy white; FT-IR (KBr); 3039, 2893, 1670, 1413, 1307 cm-1, HNMR (δ, 400MHZ, CDCl3): 8.66(s, 1H), 7.77(s, 1H), 8.36(s, 1H), 7.42(d, 2H), 6.95(s, 1H), 7.25(d, 2H) (Table 1, entry 4 fig 10, 11)
Entry |
Formic acid |
Amines |
Product |
Time(min) |
Yield (%) |
1 |
|
|
|
25 |
90 |
2 |
|
|
|
15 |
95 |
3 |
|
|
|
27 |
89 |
4 |
|
|
|
18 |
92 |
5 |
|
|
|
38 |
80 |
6 |
|
|
|
40 |
83 |
7 |
|
|
|
35 |
88 |
Table 1 N-Formylation of amines using Zeolite A in formic acid at room temperature under solvent-free conditions
X-ray diffraction patterns of H form and metal ion exchanged forms of calcinated zeolite-A is shown in Figure 3. All forms show high crystallinity nature without any amorphous phase. From diffraction studies the sharp peak is obtained at 2θ which corresponding to 24.5 for all the forms of zeolite-A. It is clear from Figure 1 we can deduce that the powder X-ray diffraction studies of H form and the metal ion exchanged forms of zeolite-A are similar to X-ray diffraction studies of its parent zeolite-A. These observations shows that zeolite-A framework does not undergo any significant structural change during the presence of the metal ion and the crystallinity was preserved, only slight change in the intensity of the bands were observed.
The crystal morphology of calcinated zeolite A, its H form and metal ion exchanged forms are shown in Figure 4. From the SEM images of zeolite-A it is observed that particles appear to have cuboidal shape with highly excellent crystal edges and the size of particle appears 2–5 μm in range. SEM image of H form and various zeolite A metal ion exchanged forms are similar to the surface micrograph of its parent zeolite A which shows that they acquire the same morphology. However small variation in the surface micrographs of SEM images of some forms is also observed and it may be due to the presence of impurity deposition on zeolite surface by metal salts and organic part of ammonium salt used during the formation of H-form and various metal ion exchanged forms respectively which could not been removed properly.
The FT-IR spectrum of calcined zeolite-A, its H form and metal ion exchanged forms are shown in Figure 5. FT-IR spectrum of calcined zeolite-A shows absorption bands at 450 cm−1 which is attributed to Si, Al-O band, and those around at 1000 cm−1 and 750 cm−1 are, respectively, attributed to symmetric and asymmetric stretches of the zeolite framework. A band peak for the OH group is clearly observed at 3400 cm−1. It is clearly observed from Figure 5 that the FT-IR spectrum of H form and metal ion exchanged forms of zeolite-A are similar to the FT-IR spectrum patterns of its parent zeolite-A. (Black line in Figure 5 shows FT-IR spectrum of parent zeolite-A)
We have developed a simple, efficient and excellent method for the synthesis of N-Formylation of amines under solvent free condition at room temperature in presence of catalyst H-zeolite A. So the model reaction was carried out between 4-Chloro aniline and Formic acid (Figure 1). Without catalyst, reaction product 25% yield was obtained when the mixture was stirred for 100 minutes. However when the catalyst (0.05g) was added to the reaction mixture, the reaction occurred in 15 minutes resulting in 95% yield. It shows that catalyst plays an efficient role in the reaction. The efficiency of model reaction was experimentally tested on various catalytic forms such as H-form of Zeolite A, Mg form of Zeolite A and K form of Zeolite A. The results are shown in Figure 6.
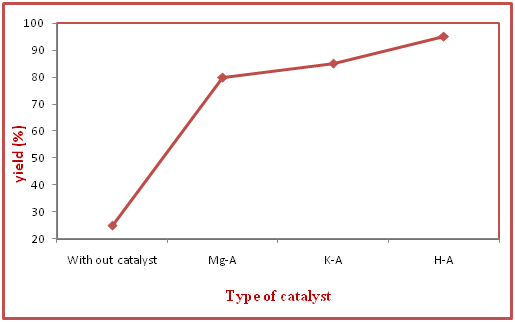
Figure 6 Effect of catalytic efficiency of H-zeolite A and ion exchanges on the synthesis of N-(4- Chlorophenyl) foramide.
aReaction condition: 4-chloroanaline (1.0 mmol), and aq. formic acid (1.2 mmol) in the presence of H-Zeolite-A (0.05 g) under solvent free condition
b Isolated yield
The experimental result of the model reaction reflects that the H-Form of Zeolite A is the most efficient catalyst for the synthesis of N-Formylation of amines as compared to various ion exchange forms of Zeolite A. The reason is that high surface and active acid sites are present in H-form of zeolite A as compared to various ion exchange forms of Zeolite A. The effect of catalyst amount of the sample reaction on the activity of the catalyst H-form of Zeolite A was observed under solvent free condition and room temperature as shown in Figure 7.
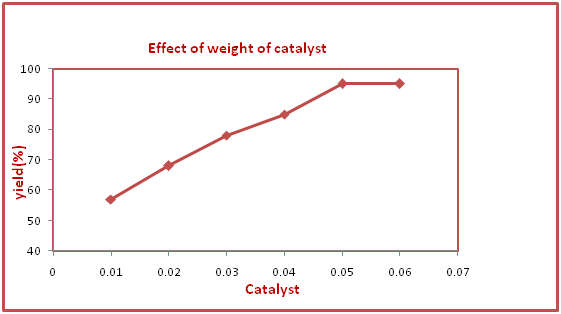
Figure 7 Effect of weight of H-Zeolite A on the synthesis of N-(4- Chlorophenyl) foramide.
aaReaction condition: 4-chloroanaline (1.0 mmol), and aq. formic acid (1.2 mmol) in the Presence of H-Zeolite-A (0.05 g) under solvent free condition
b Isolated yield
It was found that with increase in the amount of catalyst from 0.01g to 0.05g the product yield of the sampled reaction increases after which it remains constant. The reason for increasing catalyst up to 0.05g is mainly due to increase in active sites with increase in the amount of catalyst. After further increase in the catalyst, the additional acid sites cause no effect because the reactants may loss sufficient sites to bind with. Hence we used weight of catalyst as 0.05g for the rest of the reactions. To study the scope of the reaction we used various derivatives of amines to investigate two component reactions under optimized condition. We found that different amine derivatives could be utilised resulting high percentage yield. Amines possess electron donating group resulting high yield and shorter reaction time while as amines with electron withdrawing substitutents furnished moderate yield and required long reaction time. Diamines were converted into their corresponding N-Formylations resulted in good yield. The reaction time and % yield of the products are shown in Table 1.
The reusability of the catalyst is the most important tool and makes it very useful for commercial applications. After the reaction was completed , the catalyst was seperated by simple filteration and then H-form of Zeolite A was washed 2-4 times with ethylacetate and chloroform and dried in an oven at 120oC for 8 h. The catalyst was used in several runs as shown in Table 2 (Figure 8). The small change in the catalytic activity after 3 cycles is mainly due to reduce catalyst structure during recovery process. It shows that the catalyst could be recycled several times without much loss in their activity.

Figure 8 Recyclability of the catalyst: The H-zeolite A catalyst could be reused four times without any loss of its activity towards the synthesis of synthesis of N-(4-Chlorophenyl) foramide
aaReaction condition: 4-chloroanaline (1.0 mmol), and aq. formic acid (1.2 mmol) in the presence of H-Zeolite-A (0.05 g) under solvent free condition.
b Isolated yield
c Catalyst was reused four times.
Entry |
Yield (%) |
1run |
95 |
2run |
95 |
3run |
93 |
4run |
90 |
Table 2 Effect of recyclability of H-Zeolite A on the synthesis of N-(4-Chlorophenyl) foramide
In conclusion we report a simple, rapid and efficient method for the N-formylation of amines with formic acid using Zeolite A as a catalyst under solvent free condition at room temperature. The catalyst was seperated by simple filteration and recycled at least 2-4 times without loss of any catalytic activity. Shorter reaction time, high yield, easy workup and any hazardous organic solvents are some advantages of this protocol.
We are highly thankful to IIT Delhi, Jammia Milia University and IIT Patna for the characterization of the synthesized materials. Authors are also thankful to Jiwaji University for providing necessary facilities for carrying out the experimental work.
Author declares that there is no conflict of interest.

©2017 Bhat, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.