eISSN: 2379-6367


Research Article Volume 9 Issue 5
1Department of Chemistry, Faculty of Arts and Sciences, Ondokuz Mayıs University, Turkey
2Department of Chemistry, Faculty of Arts and Sciences, Giresun University, Turkey
3Department of Chemistry Education, Faculty of Education, Ondokuz Mayıs University, Turkey
Correspondence: Saim Topçu, Department of Chemistry, Faculty of Arts and Sciences, Giresun University, 28200 Giresun, Turkey
Received: August 17, 2021 | Published: September 3, 2021
Citation: Ozen T, Bal A, Topcu S, et al. Synthesis, characterization and antioxidant activities of some novel oxime derivatives. Pharm Pharmacol Int J. 2021;9(5):176-192. DOI: 10.15406/ppij.2021.09.00343
In this study, nine novel compounds were synthesized in the reaction of ω-chloro-isonitrosoacetophenone with 2-chloro-4-methylaniline, 2-chloro-5-methylaniline, 2-chloro-6-methylaniline, 3-chloro-2-methylaniline, 4-chloro-3-methylaniline, 5-chloro-2-methylaniline, 2,3-dichloroaniline, 2,4-dichloroaniline and 3,5-dichloroaniline resulting for C1-C9, respectively. Compounds C1, C4-C7 and C9 were handled in single crystalline forms, which the structures were also investigated by X-ray single crystal analysis. Compounds were evaluated for their nonenzymatic lipid peroxidation inhibition, antioxidant and antiradical activities and compared with standard agents at 5, 50, and 100μM concentrations. Compounds are exhibited good antioxidant activities and lipid peroxidation compared to standards (BHA, TBHQ, BHT and trolox). Due to the best of our knowledge for novel oxime compounds, this study is the first report of promising antioxidants to scavenge oxidative stress conditions.
Keywords: oximes, carbonyl-oxime, amide-oxime, amido-carbonyl oxime, antioxidant activity, lipid peroxidation inhibition
Reactive oxygen species (ROS) are produced in normal cellular oxygen metabolism, effective in some biological systems. The increase in ROS on the intracellular antioxidant capacity may result in a situation characterized by oxidative stress. ROS are free radicals such as hydroxyl radicals, superoxide anion radicals, and nonradical species such as singlet oxygen and hydrogen peroxide. ROS attack hepatic and extrahepatic organs and induce oxidative stress. Thus leading directly or indirectly causes degenerative diseases such as cancer, dementia, and aging.1 Cells perform many repair and protection mechanisms to remove ROS. Intracellular defense systems occur in two forms, enzymatic and non-enzymatic mechanisms.2
Antioxidants are essential compounds that reduce or neutralize the ROS, thus protecting the organisms from ROS and preventing the cells from oxidative injury. Therefore, significant research has been directed toward the identification of newly synthesized antioxidants to prevent ROS-induced damage.3,4 Recent studies exhibited that oximes and their derivatives are used for clinical, critical pharmaceutical and synthetic chemistry applications as chemical inhibitors of enzyme activities.5,6
Previous works reported that oxime derivatives possessed insecticidal, miticidal, nematocidal, antiradical, antioxidant, antiepileptic, antihyperglycemic, antimicrobial, antidote, anti-carcinogenic and anti-HIV activities.7-18 We synthesized a series of novel phenylacetamidine based amido-carbonyl oximes (C1-C9). Structures of the compounds characterized by 1H-NMR, IR spectroscopy, and X-ray crystallography. Furthermore, the goal of this work was to comparatively evaluate in vitro antioxidant properties, including total antioxidant activity, reducing power, inhibition of linoleic acid peroxidation, free radical scavenging, metal chelating, and hydrogen peroxide scavenging activity. Antioxidant activities of oxime compounds compared with standard antioxidants BHA, BHT, TBHQ, and Trolox commonly used by the food and pharmaceutical industry.
The general structure and the substitution pattern of the novel oximes are depicted in Table 1. The installation of active substituents in oxime moiety exhibits a preponderant role in the enhancement of the antioxidant activity.
Table 1 Structures of the oxime derivatives prepared in this work
All starting materials were commercially available and were reagent grade. The 1H NMR spectra were recorded on a Bruker 500 MHz NMR spectrometer, using deuterated chloroform as a solvent. IR spectra (4000–400 cm-1) were recorded on a Bruker FT-IR spectrophotometer with a sample prepared as KBr pellets. To utilize antioxidant activities were done using Thermo Scientific Evolution Array UV-Vis spectrometer in ethanol solution.
Synthesis of the compounds
Compounds were prepared from the reaction of ω-chloro isonitroso acetophenone (ω)19 with the corresponding amines by using previously reported methods.7,9,20-25 A solution of 0.01 mol aniline derivatives (1.42 g 2-chloro-4-methylaniline for C1,1.42 g 2-chloro-5-methylaniline for C2, 1.42 g 2-chloro-6-methylaniline for C3, 1.42 g 3-chloro-2-methylaniline for C4, 1.42 g 4-chloro-3-methylaniline for C5, 1.42 g 5-chloro-2-methylaniline for C6, 1.62 g 2,3-dichloroaniline for C7, 1.62 g 2,4-dichloroaniline for C8, 1.62 g 3,5-dichloroaniline for C9 was dissolved in EtOH (10ml), then the solution was added dropwise to a solution of ω-chloro isonitroso acetophenone (0.03mol, 5.5g) in ETOH (10ml), and then solid NaHCO3 (0.01mol, 0.84g) was added to the mixture. After 1 h, H2O (10ml) was added dropwise to the mixture. The precipitated product was filtered, washed with water and then recrystallised from EtOH.
The IUPAC name, structures, yields, and melting point of the compounds are tabulated in Table 1.
X-ray crystallography
The crystal data were collected by using Mo Kα, λ=0.71073 Å radiation on a Bruker Smart Apex II diffractometer. On the diffractometer, Bruker APEX2 v2014.9-026 and Bruker SAINT v8.34A software programs were used; for data collection and data reduction, respectively.27 Using OLEX2,28 the structure was solved with the Superflip29 structure solution program using Charge Flipping Methods and all non-hydrogen atoms were refined anisotropically by full-matrix least-squares methods refined with the SHELXL30 refinement package using Least Squares minimization. The hydrogen atoms were placed in geometrically idealized positions and refined as riding model (except OH proton of oxime group and the N–H protons adjacent to the oxime groups). All interaction in the solid-state was investigated by Platon analyses.31
Antioxidant and antiradical activity assays
The compounds were evaluated for their inhibition of nonenzymatic lipid peroxidation, antioxidant and antiradical activities and compared with BHA, BHT, TBHQ and Trolox at different concentrations. The evaluation of the total antioxidant activity,32,33 reducing power,34,35 Free radical scavenging activity,36 metal chelating activity,37 hydrogen peroxide scavenging activity38 and inhibition of linoleic acid peroxidation39 assays were done in 5-100 doses and compared with BHA, BHT, TBHQ and trolox at different concentrations, by lights of the literature methods. All the results are triplicates of mean ± SD.
For the compounds suitable for X-Ray in single crystals analyses, the crystallographic data, the twisting angles for defined the planes, the selected bond lengths and angles and the hydrogen bond geometries are given in Table 2-5, respectively. The functional oxime group represented by carbon number 8, first nitrogen and the second oxygen atom (C8=N1-O2) is located in the molecule's centre. According to the X-Ray results, the oxime group and the adjacent amine nitrogen atom (N2) forms a plane which were marked as P to understand and compare the positions of the groups (Figure 1). The C1 to C6 carbon atoms represents the phenyl ring (R1) adjacent to the carbonyl group. In contrast, the C9 to C14 carbon atoms forms the second phenyl ring (R2) adjacent to the amine group of the molecule. The angles between the planes are given compared in Table 3.
|
Identification code |
C1 |
C4 |
C5 |
C6 |
C7 |
C9 |
|
Empirical formula |
C15H13ClN2O2 |
C15H13ClN2O2 |
2×(C15H13ClN2O2) |
C15H13ClN2O2 |
C14H10Cl2N2O2 |
C14H10Cl2N2O2 |
|
Formula weight |
288.72 |
288.72 |
577.45 |
288.72 |
309.14 |
309.14 |
|
Temperature/K |
296.15 |
296.15 |
293(2) |
296.15 |
296.15 |
296.15 |
|
Crystal system |
triclinic |
monoclinic |
monoclinic |
triclinic |
monoclinic |
triclinic |
|
Space group |
P-1 |
C2/c |
P21/n |
P-1 |
C2/c |
P-1 |
|
a/Å |
4.9223(2) |
20.3709(8) |
10.0345(2) |
7.8097(2) |
20.1424(7) |
8.60120(10) |
|
b/Å |
9.3269(3) |
14.4755(6) |
10.4992(3) |
8.4136(2) |
14.4388(4) |
8.99180(10) |
|
c/Å |
15.6760(4) |
10.3947(5) |
27.3026(6) |
11.9910(3) |
10.3241(3) |
9.66030(10) |
|
α/° |
82.1470(10) |
90.00 |
90.00 |
92.6740(10) |
90.00 |
74.4730(10) |
|
β/° |
88.5200(10) |
107.216(2) |
96.0060(10) |
90.7470(10) |
106.0350(10) |
83.0710(10) |
|
γ/° |
85.3720(10) |
90.00 |
90.00 |
117.4000(10) |
90.00 |
78.5010(10) |
|
Volume/Å3 |
710.53(4) |
2927.8(2) |
2860.65(12) |
698.22(3) |
2885.76(15) |
703.622(13) |
|
Z |
2 |
8 |
4 |
2 |
8 |
2 |
|
ρcalcg/cm3 |
1.350 |
1.310 |
1.341 |
1.373 |
1.423 |
1.459 |
|
μ/mm‑1 |
0.271 |
0.263 |
0.269 |
0.276 |
0.451 |
0.463 |
|
F(000) |
300.0 |
1200.0 |
1200.0 |
300.0 |
1264.0 |
316.0 |
|
Crystal size/mm3 |
0.45 × 0.23 × 0.13 |
0.32 × 0.29 × 0.18 |
0.45 × 0.45 × 0.37 |
0.45 × 0.37 × 0.15 |
0.45 × 0.33 × 0.24 |
0.29 × 0.28 × 0.14 |
|
Radiation |
MoKα (λ = 0.71073) |
|||||
|
2Θ range /° |
2.62 to 66.2 |
3.5 to 66.7 |
3 to 63.98 |
3.4 to 66.76 |
3.52 to 63.74 |
4.78 to 66.06 |
|
Index ranges |
-7 ≤ h ≤ 7 |
-31 ≤ h ≤ 31 |
-14 ≤ h ≤ 14 |
-12 ≤ h ≤ 10 |
-29 ≤ h ≤ 19 |
-13 ≤ h ≤ 13 |
|
Reflections |
20280 |
22777 |
37479 |
19447 |
19129 |
20172 |
|
Independent reflections |
5349 [Rint = 0.0212 |
5671 [Rint = 0.0181 |
9805 [Rint = 0.0213 |
5344 [Rint = 0.0167 |
4922 [Rint = 0.0151 |
5268 [Rint = 0.0221 |
|
Data/restraints/parameters |
5349/0/184 |
5671/0/187 |
9805/0/367 |
5344/0/184 |
4922/0/186 |
5268/0/183 |
|
Goodness-of-fit on |
1.031 |
1.033 |
1.036 |
1.030 |
1.051 |
1.038 |
|
Final R indexes |
R1 = 0.0458 |
R1 = 0.0507 |
R1 = 0.0723 |
R1 = 0.0434 |
R1 = 0.0437 |
R1 = 0.0446 |
|
Final R indexes [all data] |
R1 = 0.0647 |
R1 = 0.0700 |
R1 = 0.0984 |
R1 = 0.0576 |
R1 = 0.0539, |
R1 = 0.0618 |
|
Largest diff. peak/hole / e Å-3 |
0.35/-0.27 |
0.29/-0.40 |
0.78/-0.32 |
0.43/-0.21 |
0.51/-0.45 |
0.59/-0.60 |
Table 2 Crystal data and structure refinement
|
Compound |
Twisting angles |
|||||
|
R1-P |
R2-P |
R1-R2 |
C=O-R1 |
C=O-P |
C=O-R2 |
|
|
C1 |
60.13(10) |
36.56(9) |
71.96(7) |
9.79(9) |
49.32(11) |
50.30(9) |
|
C4 |
63.36(9) |
37.18(7) |
89.53(8) |
25.08(10) |
37.10(9) |
44.46(9) |
|
C5 |
55.51(14) |
46.49(12) |
80.52(12) |
11.32(18) |
44.09(17) |
45.37(16) |
|
58.97(13)* |
44.49(11)* |
71.67(12)* |
7.72(13) |
49.93(13) |
45.40(11) |
|
|
C6 |
59.69(8) |
39.45(8) |
83.27(7) |
14.92(9) |
44.42(10) |
54.97(9) |
|
C7 |
60.91(10) |
34.66(9) |
86.26(9) |
23.42(11) |
36.36(10) |
45.40(10) |
|
C9 |
63.10(10) |
43.77(8) |
86.63(9) |
16.78(11) |
46.32(10) |
46.52(9) |
Table 3 The twisting angles between the oxime plane and phenyl ring planes
(standard deviation for lengths and angles are given in parentheses)
|
Comp. |
C1 |
C4 |
C5 |
C6 |
C7 |
C9 |
|
Bond Atoms |
Bond Length/Å |
|||||
|
O2-N1 |
1.4152(14) |
1.4056(12) |
1.4143(19) |
1.4134(11) |
1.4050(14) |
1.3994(13) |
|
1.4106(18)* |
||||||
|
O1-C7 |
1.2101(15) |
1.2131(14) |
1.211(2) |
1.2116(13) |
1.2086(16) |
1.2172(16) |
|
1.214(2)* |
||||||
|
N2-C8 |
1.3586(15) |
1.3569(13) |
1.355(2) |
1.3554(12) |
1.3571(16) |
1.3687(15) |
|
1.353(2)* |
||||||
|
N2-C9 |
1.4048(14) |
1.4062(13) |
1.405(2) |
1.4129(12) |
1.3970(15) |
1.3991(16) |
|
1.407(2)* |
||||||
|
N1-C8 |
1.2872(14) |
1.2896(13) |
1.287(2) |
1.2921(12) |
1.2872(15) |
1.2859(15) |
|
1.287(2)* |
||||||
|
C7-C6 |
1.4779(17) |
1.4810(17) |
1.472(3) |
1.4790(15) |
1.483(2) |
1.4744(17) |
|
1.479(2)* |
||||||
|
C8-C7 |
1.5167(16) |
1.5052(15) |
1.510(2) |
1.5169(14) |
1.5054(18) |
1.5107(16) |
|
1.513(2)* |
||||||
|
N2-H2 |
0.8172(10) |
0.8601(9) |
0.9060(14) |
0.8200(9) |
0.8405(11) |
0.8003(10) |
|
0.8391(14)* |
||||||
|
Cl1-Cx |
1.7364(13) |
1.7447(12) |
1.7392(19) |
1.7409(13) |
1.7212(14) |
1.7351(13) |
|
1.7405(19)* |
||||||
|
Cl2-Cy |
- |
- |
- |
- |
1.7266(15) |
1.7364(15) |
|
Bond Angle/° |
||||||
|
C10-C9-N2 |
119.73(11) |
121.72(10) |
118.39(16) |
117.44(10) |
117.39(11) |
119.31(12) |
|
121.43(16)* |
||||||
|
C6-C7-C8 |
120.10(10) |
118.23(9) |
120.51(15) |
117.96(9) |
118.52(11) |
119.62(10) |
|
120.36(15)* |
||||||
|
C8-N2-C9 |
126.48(10) |
128.37(9) |
126.56(14) |
127.26(9) |
128.79(11) |
123.60(10) |
|
124.89(14)* |
||||||
|
C8-N1-O2 |
110.61(9) |
110.80(9) |
110.25(14) |
110.98(8) |
111.02(10) |
111.54(10) |
|
111.13(13)* |
||||||
|
N1-C8-N2 |
123.76(11) |
122.70(10) |
124.14(15) |
124.08(9) |
122.30(12) |
124.90(11) |
|
125.32(14)* |
||||||
|
N1-C8-C7 |
114.39(10) |
112.94(9) |
115.09(15) |
113.52(8) |
113.31(11) |
113.52(10) |
|
114.15(14)* |
||||||
|
N2-C8-C7 |
120.92(9) |
122.96(9) |
119.63(15) |
121.69(8) |
122.88(11) |
120.22(10) |
|
119.65(14)* |
||||||
|
O1-C7-C8 |
117.34(11) |
118.68(11) |
116.71(17) |
118.72(10) |
118.46(13) |
117.06(11) |
|
116.33(15)* |
||||||
|
O1-C7-C6 |
122.54(11) |
122.88(11) |
122.73(17) |
123.27(10) |
122.84(13) |
123.23(11) |
|
|
|
|
123.30(15)* |
|
|
|
Table 4 Selected bond lengths and angles
(standard deviation for lengths and angles are given in parentheses)
*The asymmetric unit for C5 contains two molecules in the crystal form have different bond lengths and angles
|
C1 |
||||||
|
Donor |
Hydrogen |
Acceptor |
Bond length/Å |
Angle/° |
||
|
D |
H |
A |
d(D-H) |
d(H-A) |
d(D-A) |
D-H-A |
|
O2 |
H2 |
N11 |
0.84 |
2.00 |
2.7786(14) |
155.30(7) |
|
C10 |
Cl1 |
R22 |
|
3.5481(7) |
4.0691(16) |
94.44(5) |
|
C7 |
O1 |
R12 |
|
3.9582(12) |
4.1818(16) |
92.13(8) |
|
1-X,1-Y,1-Z, -21+X,Y,Z |
||||||
|
C4 |
||||||
|
D |
H |
A |
d(D-H) |
d(H-A) |
d(D-A) |
D-H-A |
|
O2 |
H2 |
N11 |
0.85(2) |
1.97(2) |
2.7429(13) |
150.8(19) |
|
C2 |
H2A |
R22 |
|
2.93 |
3.699(2) |
141 |
|
C15 |
H15A |
R23 |
|
2.85 |
3.7608(18) |
159 |
|
11-X,2-Y,1-Z, 21/2-X,1/2+Y,1/2-Z, 3-X,Y,1/2-Z |
||||||
|
C5 |
||||||
|
D |
H |
A |
d(D-H) |
d(H-A) |
d(D-A) |
D-H-A |
|
O2 |
H2 |
N1A1 |
0.8675(14) |
1.9405(15) |
2.758(2) |
156.54(10) |
|
*O2A |
H2B |
N12 |
0.8078(14) |
2.0480(16) |
2.802(2) |
155.12(10) |
|
R2 |
|
R23 |
|
|
3.7269(11) |
|
|
*R2 |
|
*R24 |
|
|
3.7700(11) |
|
|
1-1/2+X,1/2-Y,-1/2+Z; 21/2+X,1/2-Y,1/2+Z, 31-X,1-Y,-Z, 42-X,2-Y,-Z |
||||||
|
C6 |
||||||
|
D |
H |
A |
d(D-H) |
d(H-A) |
d(D-A) |
D-H-A |
|
O2 |
H2 |
N11 |
0.80 |
2.04 |
2.7846(11) |
154.83(6) |
|
C15 |
H15A |
R1 |
|
3.00 |
3.7799(16) |
140 |
|
R2 |
|
R22 |
|
|
3.8686(9) |
0 |
|
R2 |
|
R23 |
|
|
3.9546(9) |
0 |
|
1-X,1-Y,1-Z, 2-X,1-Y,-Z, 31-X,1-Y,-Z |
||||||
|
C7 |
||||||
|
D |
H |
A |
d(D-H) |
d(H-A) |
d(D-A) |
D-H-A |
|
O2 |
H2 |
N11 |
0.82(2) |
2.00(2) |
2.7433(15) |
150(2) |
|
C2 |
H2A |
R22 |
|
2.99 |
3.721(3) |
137 |
|
C10 |
Cl1 |
R23 |
|
3.6464(9) |
4.1206(16) |
93.29(5) |
|
1-X,1-Y,-Z, 21/2-X,1/2+Y,1/2-Z, 3-X,Y,1/2-Z |
||||||
|
C9 |
||||||
|
D |
H |
A |
d(D-H) |
d(H-A) |
d(D-A) |
D-H-A |
|
O2 |
H2 |
N11 |
0.86 |
2.0328(10) |
2.8116(14) |
150.31(7) |
|
R2 |
|
R22 |
|
|
3.7004(9) |
0 |
|
12-X,2-Y,-Z, 21-X,1-Y,2-Z |
||||||
Table 5 The hydrogen bond and interaction geometries
Maximum deviation from the planes P was detected for compound C1 (Table 3). These results indicated both phenyl rings and carbonyl group were twisted around the reference plane. The twisting was found on substituted phenyl rings (R2). The bond lengths and angels for all compounds (Table 4) were in good accord with literature analogs.7,9,20-25
The crystal structures of the crystallized compounds are given in Figure 2. The three crystals, which are C1, C6 and C9, crystallized in triclinic space group P-1. The remaining three crystals, which are C4, C5 and C7 crystallized in the monoclinic system and the space groups were found as C2/c, P21/n and C2/c, respectively.
No intra-molecular interactions were detected for the compounds in the solid-state. For all compounds, the oxime hydrogens (H2) formed intermolecular hydrogen bonds with the oxime nitrogen atoms (Figure 3, Table 5). In addition to the hydrogen bonds, the inter-molecular interactions were explained and shown in Figures 3-8.
The chlorine (Cl1) and carbonyl oxygen (O2) atoms of compound C1 formed π interactions with the R2 and R1 phenyl rings, respectively (Figure 3, Table 5).
The C2 and C15 atoms of compound C4 formed C-H∙∙∙π interactions with the R2 phenyl rings at 1/2-X,1/2+Y,1/2-Z and -X,Y,1/2-Z, respectively (Figure 4, Table 5).
The chlorine and methyl-substituted phenyl rings (R2) of compound C5 formed π∙∙∙π interactions with symmetry-related the R2 phenyl rings at 1-X,1-Y,-Z, 2-X,2-Y,-Z, respectively (Figure 5., Table 5).
The chlorine and methyl-substituted phenyl rings (R2) of compound C6 formed π∙∙∙π interactions with symmetry-related the R2 phenyl rings at -X,1-Y,-Z, 1-X,1-Y,-Z, respectively (Figure 6., Table 5).
The Cl1 atom of compound C7 formed π interactions with the R2 phenyl ring. The C2 atom of the compound also formed C-H∙∙∙π interactions with the R2 phenyl ring at 1/2-X,1/2+Y,1/2-Z, via its H2A atom (Figure 7., Table 5).
The substituted phenyl rings (R2) of compound C9 formed π∙∙∙π interactions with symmetry-related the R2 phenyl rings at 1-X,1-Y,2-Z (Figure 8., Table 5).
1H NMR spectra of the compounds
A representative H-NMR spectrum of C8 is shown in Figure 9. In the 1H NMR spectral data of the compounds given in Table 6, the OH proton of the oxime group and the N–H protons were seen very close to each other. While the peaks for the OH proton of oxime groups are observed at 8.05–8.02 ppm, the N–H protons adjacent to the oxime groups resonate at 8.00–8.18 ppm. The aromatic C–H protons resonate at 7.84–6.02 ppm while aliphatic C–H protons at 2.27–2.02 ppm. These results are in good agreement with those of known oximes24,25,40-43 and coincide with handled structures by the single crystal data.21-23,44-48
|
Comp. |
OH |
NH |
Aromatic |
CH3 |
|||||
|
C1 |
8.03 |
8.01 |
7.61 |
7.47 |
7.11 |
7.01 |
6.72 |
6.57 |
2.23 |
|
C2 |
8.02 |
8 |
7.59 |
7.45 |
7.07 |
6.91 |
6.83 |
6.68 |
2.37 |
|
C3 |
8.2 |
8.18 |
7.84 |
7.67 |
7.56 |
7.54 |
7.47 |
6.02 |
2.02 |
|
C4 |
8.02 |
8 |
7.59 |
7.46 |
7.06 |
6.94 |
6.78 |
6.73 |
2.27 |
|
C5 |
8.03 |
8 |
7.59 |
7.45 |
7.13 |
6.85 |
6.7 |
6.67 |
2.22 |
|
C6 |
8.02 |
8 |
7.58 |
7.45 |
7.18 |
7.18 |
6.72 |
6.6 |
2.14 |
|
C7 |
8.02 |
8 |
7.61 |
7.46 |
7.33 |
7.23 |
7.02 |
6.7 |
- |
|
C8 |
8.05 |
8.03 |
7.61 |
7.47 |
7.33 |
7.1 |
6.97 |
6.65 |
- |
|
C9 |
8.05 |
8.03 |
7.63 |
7.49 |
7.08 |
6.98 |
6.71 |
- |
- |
Table 6 1H NMR spectra of the compounds in CDCl3
In the IR spectra of the compounds, bands at 3319-3392, 3123-3248, 1662-1682, 1603-1658, 1327-1385 and 950-999 cm-1 belong to N–H, O–H, C=O, C=N, –C–N– and N–O vibrations, respectively. These values are in accord with those of previously reported analogs of the compounds.24,25 The IR spectra data of compounds are given in Table 7.
|
Comp. |
N-H |
O-H |
C=O |
C=N |
-C-N- |
N-O |
|
ω |
- |
3277 |
1659 |
- |
1036 |
|
|
C1 |
3392 |
3234 |
1678 |
1636 |
1374 |
950 |
|
C2 |
3385 |
3227 |
1682 |
1634 |
1379 |
964 |
|
C3 |
3319 |
3150s |
1667 |
1613 |
1327 |
999 |
|
C4 |
3378 |
3180 |
1681 |
1603 |
1379 |
964 |
|
C5 |
3328 |
3153 |
1668 |
1622s |
1367 |
969 |
|
C6 |
3380 |
3203 |
1680 |
1634 |
1376 |
956 |
|
C7 |
3358 |
3183 |
1679 |
1611 |
1371 |
969 |
|
C8 |
3345 |
3123 |
1672 |
1636 |
1385 |
953 |
|
C9 |
3367 |
3248 |
1662 |
1658s |
1356 |
990 |
Table 7 The some IR spectra data of compounds
Total antioxidant activity
Total antioxidant activity assay is based on the reduction of phosphate-Mo (VI) to phosphate Mo (V) by the oximes and subsequent formation of a green-colored phosphate/Mo (V) complex and compared with those of BHA, TBHQ, BHT, and Trolox at 5-100μM as positive controls. This method is routinely applied in the samples to evaluate the total antioxidant capacity.33,49 Generally, a strong electron-withdrawing substituent in the phenyl ring increase antioxidant activity.9,50 The antioxidant capacities of the oximes were determined for 5-100μM concentrations and shown in Figure 10. The results showed that different substituents and concentrations affected the total antioxidant activities of newly synthesized oximes. The increasing of the chlorine and methyl groups to oxime benzene caused the exposure to show differences in antioxidant activities. The p-position of -methyl and -chlorine attached to the benzene ring exhibited the best total antioxidant activity in the C6 and C2 compounds. Compound C6 exhibited better reduction from Mo(VI) to Mo(V) than standards due to the presence of -5 chloro and -2 methyl groups. Compounds C2 and C4 showed high activity according to BHA at 100 μM. The increase in the concentration of compounds and standards exhibited a significant increase in the antioxidant activities (p < 0.05). The trend observed in absorbance values at 695 nm were C6 > TBHQ > trolox > BHT > C2 > C4 > BHA > C5 > C9 > C8 > C7 > C1 >C3 at 100 μM.
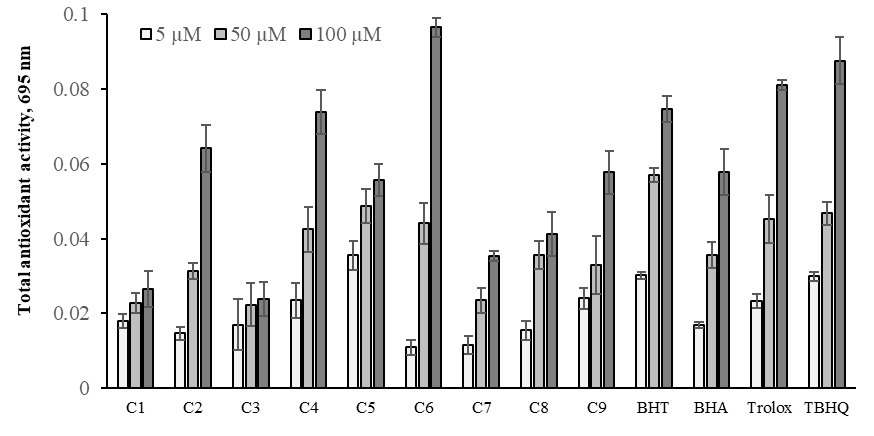
Figure 10 Total antioxidant activity of the oxime derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant activity, p<0.05. Each value represents means±SD (n=3).
Reducing power
The Fe3+ is the relatively biological inactive form of iron and reduced to the active Fe2+.51 Fe2+ can be oxidized back to Fenton Reaction with a production of ˙OH or Haber–Weiss Reaction with O2.-. The presence of reductants causes the reduction of the Fe3+-ferricyanide complex to the Fe2+ recorded by measuring Perl’s Prussian blue at 700 nm.52 The reducing power abilities of oximes were measured at concentrations (5-100 μM)35 and compared standards (BHA, BHT, TBHQ, trolox). An increase in absorbance of the reaction mixture may indicate an increase in the reducing capacity due to an increase in the formation of the complex, Fe4[Fe(CN−)6]3. The reducing power exerts antioxidant action by donating of a hydrogen atom to break the free radical chain.53 All synthesized compounds and standards are depicted in Figure 11. The reducing power capacities were not significantly dose-dependent in the test. As shown in Table 2, compounds C5, C6, C8 and C1 have the most powerful ferric ion (Fe3+) reducing power. The reducing power capacity of oxime compounds were in the following order: C5 > C6 > C8 > C1 > BHA > C4 > BHT > C9 > C2 > C7 > trolox > TBHQ > C3 at higher concentration and showed moderate reducing power activity.
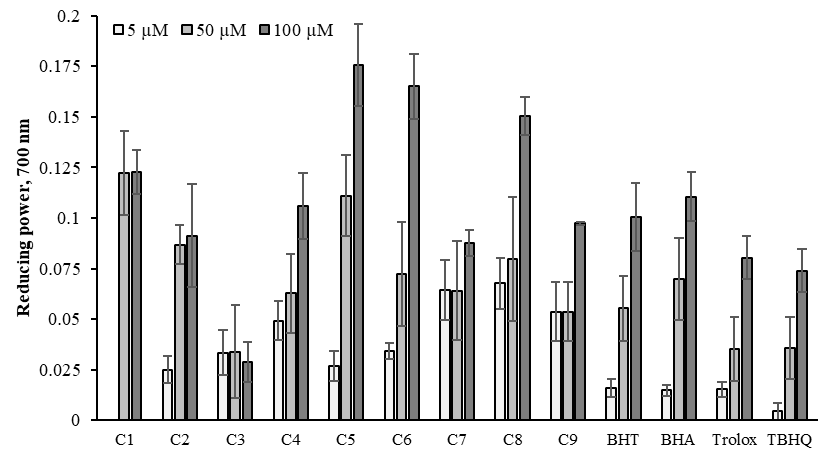
Figure 11 Reducing power of the oxime derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant capacity, p<0.05. Each value represents means ± SD (n = 3).
As can be seen in Figure 2, compound 5j and 5k has the most powerful ferric ion (Fe3þ) reducing capability.
Free radical scavenging activity
The DPPH free radical (DPPH˙) is a stable and well-known radical used in medicine, food, and health scientific research. DPPH˙ scavenging activity determination method is cheap, simple, and, fast and therefore, it is used to analyze of many synthetic and natural products. The free radical scavenging test determined the antiradical activities of the compounds. The action of antioxidant molecules causes this brilliant color. The antioxidant molecules convert the DPPH˙ to DPPH-H by transferring hydrogen source or electron. The active pink colored DPPH˙ will be removed with a light yellow color conversion and is measured at 517nm.54 The novel compounds exhibited lower free radical scavenging activity than standards (Figure 12). Based on the observed results, C5 compound exhibited a powerful free radical scavenger due to electron donor substituents (C5: 59.11%). C1, C6 and C2 compounds have better free radical scavenging activity than BHT (56.14, 55.48 and 55.07 %, respectively). The effects of -methoxy and –hydroxyl groups on the phenyl ring of oximes in ascending order were found to be: trolox > TBHQ > BHA > C5 > BHT > C1 > C6 > C2 > C7 > C4 > C8 > C9 > C3 at higher concentration, significantly (p< 0.05).
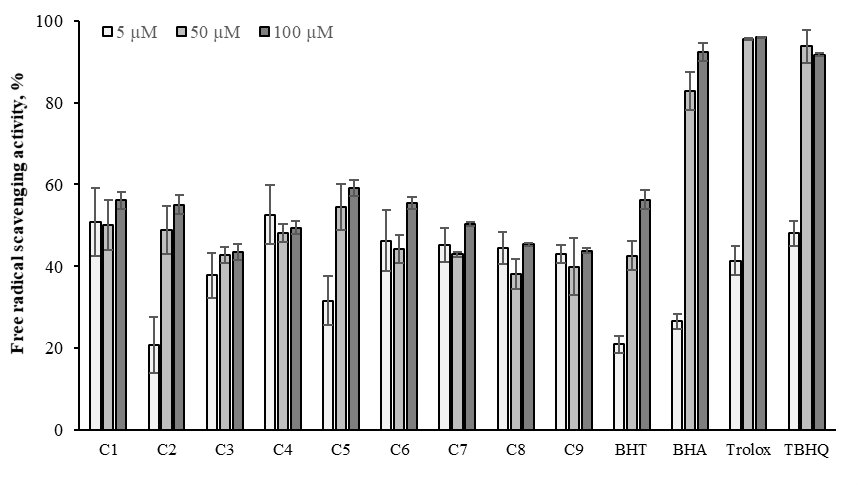
Figure 12 Free radical scavenging activity of the oximes derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant activity, p<0.05. Each value represents means ± SD (n = 3).
Metal chelating activity
Transition metals (iron, vanadium, nickel, copper, cobalt, chromium, arsenic, cadmium) play an important role in the decomposition reaction of H2O2 and lead to the formation of O2˙- and ˙OH.55 These free radicals may accelerate protein damage, lipid peroxidation, and DNA damage. Additionally, active transition metals transfer a single electron in oxidation reactions. Some antioxidant compounds inhibit oxidation by Fe2+ chelating activity, reduce redox potential and stabilize metal oxide. Chelating new agents are effective as synthetic antioxidants due to inhibiting the transition metal-dependent and process stabilizing the oxidized form of the active metal ion.34,56 Chelating activities of oxime derivatives were compared to four chelating standards as BHT, BHA, TBHQ and trolox (Figure 13). The concentration of compounds and standards exhibited a significant increase in the metal chelating activities (p<0.05). C2 exhibited higher metal-chelating activity than novel oximes and standards at 100μM. The increasing order of the metal chelating activity of the samples displayed in the following order of C2 > BHT > C3 > TBHQ > C4 > C7 > BHA > C9 > trolox > C1 > C6 > C8 > C5 that were 24.45, 23.18, 20.95, 19.65, 18.99, 18.22, 16.24, 14.60, 12.05, 11.38, 11.34, 6.09, 4.96%, at 100μM, respectively.
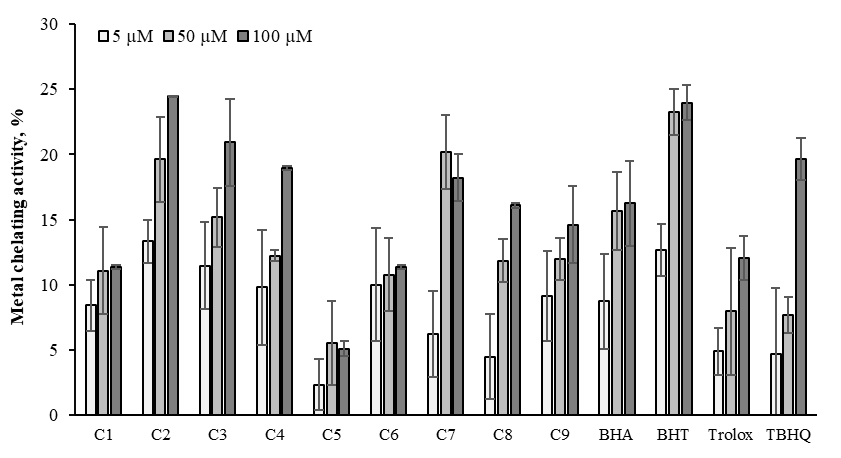
Figure 13 Metal chelating activity of the oximes derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant activity, p<0.05. Each value represents means ± SD (n = 3).
Hydrogen peroxide scavenging activity
H2O2 forms in vivo by antioxidant enzymes in biological systems and indicates a precursor to producing the ·OH. The ·OH can cause tissue damage and react with most bimolecular cell death and cross cell membrane.57 Thus, the scavenge of ·OH is crucial for the elimination of cells. Therefore, we investigated the hydrogen peroxide scavenging activity of the newly synthesized derivatives compared with standards (BHA, TBHQ, BHT and trolox) at the same dose (Figure 14). The hydrogen peroxide scavenging activities of the compounds and standards are strongly dependent on the concentration (p<0.05). C2 and C5 exhibited effective H2O2 scavenging activity higher than standards at the same dose. The scavenging activity values of H2O2 were as follows: C2 > C5 = TBHQ > C6 > C8 > C4 > BHT > C9 > C3 > C1 > Trolox > C7 > BHA which were 64.81, 57.41, 57.41, 45.56, 42.59, 33.15, 31.48, 29.81, 26.85, 25.56, 24.07, 16.48 and 14.81% at 100 μM, respectively.
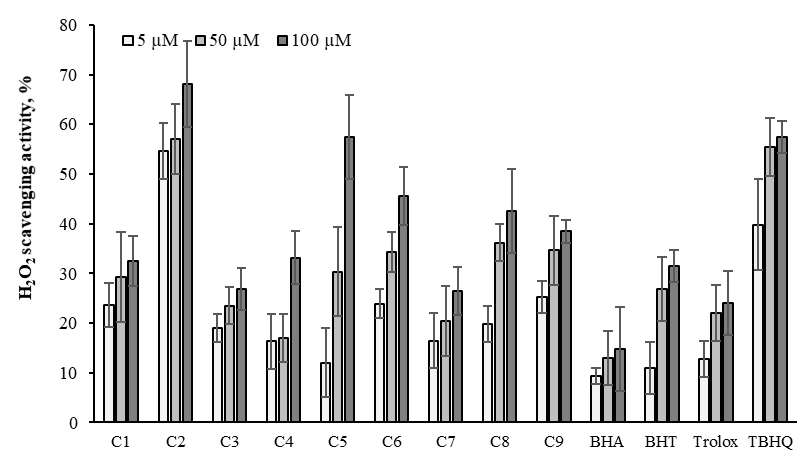
Figure 14 H2O2 scavenging activity of the oximes derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant activity, p<0.05. Each value represents means ± SD (n = 3).
Inhibition of linoleic peroxidation assay
Several chemical and physical phenomena can initiate oxidation, which proceeds continuously in a suitable substrate(s) until a blocking defense mechanism occurs. Target substances include oxygen, polyunsaturated fatty acids, phospholipids, cholesterol, and DNA.58 Lipids and lipid-containing materials are tending to peroxidation during processing and storage. Lipid peroxidation, a complex free radical chain process, involves an array of radicals and is measured by the amount of peroxide and the primary lipid oxidation product produced during the initial stages of oxidation.59 The oxime derivatives tested the nonenzymatic linoleic peroxidation at 5-100μM. Oxime derivatives dose-dependently inhibited the linoleic peroxidation induced by Fe2+. The activity extent of oximes and standards was in the comparable level at 5-100μM concentration and exhibited a significant increase in the inhibition of linoleic peroxidation (p<0.05) (Figure 15). C3 (88.34%), C9 (85.70%) and C6 (74.93%) had the higher linoleic acid peroxidation inhibition activity at 100 μM than trolox and the results were found in order of TBHQ > C3 > C9 > C6 > trolox > C8 > BHA > BHT > C4 > C7 > C1 > C5 > C2. Also, C3, C9 and C6 compounds can inhibit free radical-induced chain reactions and biological damage-causing lipid peroxidation.

Figure 15 Nonenzymatic linoleic acid peroxidation level of the oximes derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant activity, p<0.05. Each value represents means ± SD (n = 3).
Statistical analysis
The data were presented as the mean ± standard deviation (S.D.). Statistical analysis for antioxidant activities was analyzed using one-way ANOVA followed by Tukey’s HSD test with α=0.05. These assessments were conducted using SPSS (20.0) software. All assays were performed in triplicate.
|
Samples |
Antioxidant activity, 695 nm |
Reducing Power, 700 nm |
||||
|
5μM |
50μM |
100μM |
5μM |
50μM |
100μM |
|
|
C1 |
0.0180±0.0019a |
0.0228±0.0027a |
0.0264±0.0048a |
0.0000±0.0000a |
0.1222±0.0207b |
0.1229±0.0108b |
|
C2 |
0.0147±0.0018a |
0.0314±0.0021b |
0.0742±0.0162c |
0.0250±0.0168a |
0.0868±0.0096b |
0.0913±0.0054b |
|
C3 |
0.0170±0.0068a |
0.0224±0.0059a |
0.0239±0.0045a |
0.0335±0.0111a |
0.0340±0.0229a |
0.0289±0.0097a |
|
C4 |
0.0180±0.0091a |
0.0425±0.0060b |
0.0738±0.0059c |
0.0492±0.0097a |
0.0627±0.0195a |
0.1060±0.0165b |
|
C5 |
0.0356±0.0039a |
0.0487±0.0045b |
0.0556±0.0044b |
0.0267±0.0075a |
0.1110±0.0210b |
0.1757±0.0202c |
|
C6 |
0.0110±0.0020a |
0.0541±0.0149b |
0.0965±0.0065c |
0.0343±0.0038a |
0.0722±0.0258a |
0.1650±0.0160b |
|
C7 |
0.0116±0.0025a |
0.0235±0.0034b |
0.0353±0.0014c |
0.0645±0.0150a |
0.0641±0.0246a |
0.0876±0.0063a |
|
C8 |
0.0155±0.0026a |
0.0356±0.0037b |
0.0413±0.0059b |
0.0677±0.0126a |
0.0795±0.0307a |
0.1505±0.0095b |
|
C9 |
0.0240±0.0029a |
0.0330±0.0078a |
0.0544±0.0116b |
0.0538±0.0146a |
0.0643±0.0304a |
0.0974±0.0007a |
|
BHA |
0.0169±0.0007a |
0.0356±0.0036a |
0.0578±0.0062c |
0.0148±0.0027a |
0.0699±0.0204b |
0.1105±0.0120c |
|
BHT |
0.0302±0.0010a |
0.0569±0.0019a |
0.0746±0.0034c |
0.0159±0.0044a |
0.0553±0.0159b |
0.1005±0.0168c |
|
TBHQ |
0.0300±0.0012a |
0.0467±0.0029a |
0.0842±0.0120c |
0.0045±0.0042a |
0.0357±0.0152b |
0.0740±0.0107c |
|
TRX |
0.0232±0.0019a |
0.0453±0.0063a |
0.0810±0.0013c |
0.0153±0.0037a |
0.0352±0.0160a |
0.0803±0.0107b |
Table 8 Total antioxidant activity and reducing power of the oxime derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant inhibition, p <0.05. Each value represents means ± SD (n = 3)
Note: Different superscripts in the same column express significant differences (P <.05)
Abbreviations: BHT, butylated hydroxytoluene; BHA, butylated hydroxyanisol; TBHQ, t-butyl-hydroxyquinone; TRX, trolox
|
Samples |
Free radical scavenging activity, % |
Metal chelating activity, % |
|||||
|
5 μM |
50 μM |
100 μM |
5 μM |
50 μM |
100 μM |
||
|
Compounds |
C1 |
50.75±8.28a |
50.02±6.14a |
56.14±2.03a |
8.40±1.93a |
11.05±3.34a |
11.38±0.15a |
|
C2 |
20.79±6.94a |
48.89±5.92b |
55.07±2.36b |
13.32±1.63a |
19.63±3.25ab |
24.45±0.04b |
|
|
C3 |
37.86±5.47a |
42.76±1.88a |
43.48±1.98a |
11.47±3.35a |
15.16±4.24a |
20.95±6.54a |
|
|
C4 |
52.57±7.21a |
48.15±2.21a |
49.41±1.60a |
9.80±4.41a |
12.21±0.42a |
18.99±0.16b |
|
|
C5 |
31.61±5.91a |
54.52±5.66b |
59.11±1.88b |
2.32±1.98a |
5.53±3.25a |
5.09±0.58a |
|
|
C6 |
46.27±7.38a |
44.26±3.47a |
55.48±1.44a |
10.01±4.32a |
10.76±2.81a |
11.34±0.16ab |
|
|
C7 |
45.16±4.20ab |
42.91±0.60a |
50.34±0.59b |
6.21±3.29a |
20.21±5.83ab |
18.22±1.80b |
|
|
C8 |
44.51±3.82a |
38.19±3.71a |
45.39±0.38a |
4.46±3.28a |
11.85±1.64a |
16.09±0.21b |
|
|
C9 |
43.05±2.20a |
39.97±6.86a |
43.84±0.54a |
9.13±5.49a |
11.94±1.62a |
14.60±2.95a |
|
|
Standards |
BHA |
26.54±1.90a |
82.87±4.60b |
92.41±2.19c |
8.71±3.66a |
15.66±3.02a |
16.24±3.26a |
|
BHT |
20.93±2.14a |
42.55±3.56b |
56.33±2.23c |
12.66±1.99a |
23.25±1.79b |
23.98±4.33b |
|
|
TBHQ |
48.06±3.09a |
93.75±4.10b |
94.24±2.02b |
4.66±5.12a |
7.65±1.38a |
19.65±1.65b |
|
|
TRX |
41.34±3.54a |
95.51±0.25b |
95.95±0.08b |
4.88±1.82a |
7.96±4.87a |
12.05±1.67a |
|
Table 9 Free radical scavenging and metal chelating activity of the oximes derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant inhibition, p<0.05. Each value represents means ± SD (n = 3)
Note: Different superscripts in the same column express significant differences (P <.05)
Abbreviations: BHT, butylated hydroxytoluene; BHA, butylated hydroxyanisol; TBHQ, t-butyl-hydroxyquinone; TRX, Trolox
|
Samples |
H2O2 scavenging activity, % |
Nonenzymatic linoleic peroxidation, % |
|||||
|
5 μM |
50 μM |
100 μM |
5 μM |
50 μM |
100 μM |
||
|
Compounds |
C1 |
23.70±4.46a |
29.26±8.98a |
32.56±5.01a |
28.70±8.32a |
29.26±8.98a |
25.56±3.85b |
|
C2 |
54.63±5.59a |
57.04±8.98a |
68.15±8.63a |
54.63±5.59a |
55.30±10.56b |
64.81±11.56c |
|
|
C3 |
19.07±2.85a |
23.52±3.70a |
26.85±4.24a |
24.07±8.49a |
23.52±3.70b |
26.85±4.24c |
|
|
C4 |
16.30±5.59a |
17.04±4.79b |
33.15±5.28b |
16.30±5.59a |
17.04±4.79a |
33.15±5.28b |
|
|
C5 |
12.04±6.99a |
30.37±8.91a |
57.41±8.49b |
12.04±6.99a |
23.70±14.07b |
57.41±8.49c |
|
|
C6 |
23.89±2.89a |
34.26±4.02a |
45.56±5.77b |
23.89±2.89a |
30.93±8.36a |
45.56±5.77b |
|
|
C7 |
16.48±5.56a |
20.37±6.99a |
26.48±4.85a |
19.81±8.68a |
20.37±6.99b |
16.48±5.56c |
|
|
C8 |
19.81±3.70a |
36.19±3.76b |
42.59±8.49b |
19.81±3.70a |
41.85±6.12b |
42.59±8.49c |
|
|
C9 |
25.19±3.21a |
34.63±6.90ab |
38.48±2.36b |
35.19±3.21a |
34.63±6.90b |
29.81±1.79c |
|
|
Standards |
BHA |
9.37±1.67a |
12.96±8.49a |
14.81±8.49a |
13.70±7.56a |
12.96±8.49b |
14.81±8.49c |
|
BHT |
10.93±5.28a |
26.85±6.39b |
31.48±3.21b |
10.93±5.28a |
23.52±11.71b |
31.48±3.21c |
|
|
TBHQ |
39.81±9.20a |
55.37±5.84ab |
57.41±3.21b |
39.81±9.20a |
55.37±5.84b |
57.41±3.21c |
|
|
TRX |
12.78±3.64a |
22.04±5.56a |
24.07±6.42a |
16.11±6.41a |
22.04±5.56b |
24.07±6.42c |
|
Table 10 H2O2 scavenging activity and nonenzymatic linoleic peroxidation of the oximes derivatives and standards at 5, 50 and 100μM. Where-corresponds to significant inhibition, p<0.05. Each value represents means ± SD (n = 3)
Note: Different superscripts in the same column express significant differences (P <.05)
Abbreviations: BHT, butylated hydroxytoluene; BHA, butylated hydroxyanisol; TBHQ, t-butyl-hydroxyquinone; TRX, trolox
In conclusion, the structures of the synthesized oxime compounds were confirmed by X-Ray crystallography, H-NMR spectrometry, and IR spectroscopy. It was observed that oxime hydrogens (H2) form intermolecular hydrogen bonds with nitrogen atoms in the oxime group of the neighboring molecule. In addition to hydrogen bonds, intermolecular interactions in the solid state were determined.
We have confirmed that the phenyl ring system containing chloro and methyl substitutions are found to exhibit good antioxidant activities and lipid peroxidation compared to standards effects (BHA, TBHQ, BHT and trolox). The interactions of -chloro and –methyl groups in the phenyl ring of C6 compound make a reduction of improvement from Mo(VI) to Mo(V) in total antioxidant activity. C3, C9, and C6 oximes were exhibited as the most potent inhibition of linoleic acid peroxidation. The results of reducing power, free radical scavenging, metal chelating and hydrogen peroxide scavenging activity at 5-100 μM concentrations showed significant reactive for all tested oxime compounds. The results obtained by testing of antioxidant activity purposed that the oxime derivatives might be useful as potential compounds for the new antioxidant agents preventing oxidation or a source for food and pharmaceutical with chemical structures.
None.
Authors declare that there is no conflict of interest.

©2021 Ozen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.