eISSN: 2379-6367


Research Article Volume 10 Issue 6
1Laboratory of Biopharmacy, School of Pharmaceutical Sciences, University of Shizuoka, Japan
2Healthcare Business Headquarters, Ishihara Sangyo Kaisha, Ltd., Japan
Correspondence: Satomi Onoue, Laboratory of Biopharmacy, School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka, 422-8526, Japan, Tel +81-54-264-5630, Fax +81-54-264-5636
Received: December 15, 2022 | Published: December 26, 2022
Citation: Onoue S, Higuchi K, Yamane C, et al. Species differences in the biopharmaceutical properties of fuzapladib sodium monohydrate in rats, cats, and dogs. Pharm Pharmacol Int J. 2022;10(6):225-228. DOI: 10.15406/ppij.2022.10.00391
The present study aimed to characterize biopharmaceutical properties of fuzapladib sodium monohydrate, a new animal drug for acute pancreatitis, with a focus on species and gender differences. Fuzapladib sodium monohydrate (2 mg/kg of body weight) was administered subcutaneously or intravenously in male and female rats, cats, and dogs, and pharmacokinetic behavior was monitored by chromatographic analyses. With use of liver S9 fractions from each species, metabolism kinetics for fuzapladib were measured in vitro, with or without potent CYP inhibitors. After subcutaneous administration of fuzapladib sodium monohydrate (2 mg/kg), fuzapladib was rapidly absorbed in all the species tested (male), with Cmax values of 3.2 (rats), 6.6 (cats), and 14.7 µg/mL (dogs). Clearance of fuzapladib was significantly different between the species as evidenced by variable apparent elimination kinetics (male): 2.1, 0.30, and 0.13 h-1 for rats, cats, and dogs, respectively. Gender differences in pharmacokinetic behavior were not significant. Cytochrome P450 (CYP) inhibitors reduced metabolism of fuzapladib in liver S9 fraction from all three species, with significant differences in inhibitory patterns between species and various CYP inhibitors. Pharmacokinetic behavior of fuzapladib is variable depending on species, and this could be explained in part by metabolic transition.
Keywords: animal drug, fuzapladi sodium monohydrate, metabolism, pharmacokinetics, species difference
Acute pancreatitis is known to induce multi-organ dysfunction due to activation of proteolytic enzymes,1 and neutrophils and macrophages also play a key role in inflammatory responses in acute pancreatitis.2 Phospholipase A2 (PLA2), a lipolytic enzyme, is a key regulatory enzyme in the generation of prostanoids,3 and a significant increase in type II PLA2 activity has been thought to contribute to the pathogenesis of acutepancreatitis.4 Previously, fuzapladib sodium monohydrate (IKV-741,N-[2-(ethylsulfonylamino)-5-(trifluoromethyl)-3-pyridinyl]cyclohexanecarboxamide mono sodium salt monohydrate) was developed as a potent phospholipase A2 inhibitor.5 Fuzapladib sodium monohydrate can also act as a leucocyte function-associated activation (LFA-1) inhibitor that has been shown to effectively block neutrophil extravasation in experimental animals.2 Due to these biological functions, fuzapladib could reduce pancreatic hemorrhage and necrosis in experimental animal models of acute pancreatitis.6-9 In 2018, fuzapladib sodium monohydrate received approval from the Japanese Ministry of Agriculture, Forestry and Fisheries for the improvement of clinical signs in the acute phase of pancreatitis in dogs. The Japanese approved label dose is of 0.4 mg/kg via IV injection once daily for five days.
Acute pancreatitis is recognized in other species,10 and has been diagnosed with increasing frequency in cats.11,12 Fuzapladib sodium monohydrate may be of value in the treatment of acute pancreatitis for species other than dogs, although the main challenge would be to determine a suitable dosing regimen, including dose rate, dosing route, inter-dosing interval, and duration of treatment, for other species. Fundamental to dose characterization of a novel drug in a new species is an understanding of its pharmacokinetics. The dosing regimen for a drug in a given species might depend on the anatomy, biochemistry, physiology and behavior.13 In particular, species and gender differences in pharmacokinetic behavior would have major impact on the definition of dosing regimen fuzapladib sodium monohydrate; however, far less is known about the biopharmaceutical characteristics of fuzapladib sodium monohydrate in small animals.
The present study was undertaken to characterize pharmacokinetic behavior of fuzapladib sodium monohydrate in male and female rats, cats and dogs after intravenous or subcutaneous administration. To elucidate the metabolic pathway, fuzapladib sodium monohydrate was incubated with liver S9 fractions obtained from rats, cats and dogs, and the inhibitory effects of potent cytochrome P450 (CYP) inhibitors were assessed.
Materials
Fuzapladib sodium monohydrate was donated from ISHIHARA SANGYO KAISHA, Ltd. (Osaka, Japan). Alpha-naphthoflavone, ticlopidine, phenytoin, quinidine, disulfiram, ketoconazole, and β-nicotinamide-adenine dinucleotide phosphate (NADPH) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). S9 fractions from rats, dogs, and cats were obtained from Funakoshi Co., Ltd. (Osaka, Japan). Methanol and acetonitrile (liquid chromatography grade) was bought from FUJIFILM Wako Pure Chemical Corp. (Tokyo, Japan). Other chemicals were purchased from commercial sources.
Animals
All animal experiments were carried out according to the guidelines approved by the Institutional Animal Care and Ethical Committee of the University of Shizuoka. Male and female Sprague-Dawley rats (Japan SLC, Shizuoka, Japan) were provided with food and water at all times. Their housing complied with animal welfare guidelines for size and enrichment and maintained a 12-h dark/light cycle under 24±1°C (75’ F) and 55±5% relative humidity. Male and female beagle dogs (9–55 months old, 7.28–10.0 kg, Kitayama Labes, Nagano, Japan) and hybrid cats (7–60 months old, 2.40–5.08 kg, Kitayama Labes, Nagano, Japan and Shiraishi Animals, Saitama, Japan) were housed and maintained under similar conditions.
Pharmacokinetic study
Four subjects of each species received a single intravenous or subcutaneous administration of saline solution or fuzapladib sodium monohydrate at a dose of 2 mg/kg. Saline solution of fuzapladib sodium monohydrate, stored at 4°C, was found to be stable for at least 10 days. After the subcutaneous administration of fuzapladib sodium monohydrate, blood samples were collected at 5, 10, 15, 30, 60, 120, 240, 360, and 480 min after administration. Animals were awake with no anesthesia/analgesic agents used during blood sampling. The blood samples were centrifuged to obtain plasma samples, and plasma samples were frozen at -30°C until measurement of fuzapladib concentration.
Chromatographic analysis of fuzapladib
Plasma samples were deproteinized using methanol containing internal standard (IS) and fuzapladib concentration was determined using ultra-performance liquid chromatography (UPLC) with electrospray ionization mass spectrometry (ESI-MS) and high-performance liquid chromatography (HPLC) with UV detection.
For rat plasma samples, Waters Acquity UPLC system (Waters Corporation, Milford, Massachusetts) connected with MassLynx software was employed to measure the plasma concentrations of fuzapladib. Acquity UPLC BEH C 18 (particle size: 1.7 µm, column size: 2.1×50 mm; Waters) was used for the separation, and the column temperature was maintained at 65°C. The standard and plasma samples were separated using a gradient mobile phase consisting of methanol containing 0.1% formic acid (A) and 0.1% formic acid (B) with the flow rate of 0.25 mL/min. The gradient conditions of the mobile phase were 0–0.5 min, 60% A; and 0.5–3.5 min, 60–80% A. Peaks for IS (tranilast) and fuzapladib were detected at the retention times of 1.8 and 2.6 min, respectively. Detection of tranilast and fuzapladib was carried out using single ion monitoring for specific m/z 326 and 378, respectively.
For the plasma samples obtained from dogs and cats, Waters Acquity UPLC system (Waters Corporation), consisting of solvent delivery pump, auto injector, system controller, column oven (40°C) and UV detector (278 nm), was used to quantify the plasma levels of fuzapladib. L-column ODS (particle size: 5 µm, column size: 4.6×150 mm; CERI) was selected to separate fuzapladib. The standard and plasma samples were separated using a gradient mobile phase consisting of acetonitrile (A) and 0.1% phosphoric acid (B) with the flow rate of 1.0 mL/min. The gradient conditions of the mobile phase were 0–8 min, 30% A; and 8–12 min, 30–90% A. Peaks for indole (IS for dog plasma) and pyrene (IS for cat plasma) and fuzapladib were detected at the retention times of 11.6, 18.6 and 13.3 min, respectively.
Inhibition of metabolism with CYP inhibitors
The inhibition assays of cytochrome P450 using liver S9 fraction from rat, dogs and cats were conducted in accordance with previously reported procedure with some modifications.14,15 As the major CYP inhibitors for CYP 1A, 3A, 2B, 2C, 2D, and 2E, α-naphthoflavone, ketoconazole, ticlopidine, phenytoin, quinidine, and disulfiram were selected, respectively.16-18 Inhibitors were dissolved in 100 mM Tris-HCl buffer (pH7.4) with 10% DMSO. The final concentration of DMSO in the reaction mixture was lower than 1%. All incubations were carried out in triplicate. Briefly, 240 µL of the reaction medium, containing S9 fraction (2 mg-protein/mL for rat; and 5 mg-protein/mL for dog and cat), and 10 mM of each specific inhibitor, was pre-incubated for 5 min at 37°C. After the pre-incubation, 30 µL of 400 ng/mL fuzapladib sodium monohydrate and 30 µL of 10 mM NADPH solution were prepared in 100 mM Tris-HCl buffer (pH7.4), added to the reaction medium (final volume, 300 µL), and the reaction mixture was incubated for 60 min at 37°C. The reactions were stopped with 900 µL of ice-cold methanol, containing 500 ng/mL of tranilast as the IS for UPLC assay. The solutions were centrifuged at 10,000×g for 10 min, and the supernatants were filtered with 0.20 µm filter (Millex®-LG, Millipore Co., Ltd., Billerica, MA, USA). The residual amount of fuzapladib was assayed by UPLC/ESI-MS system as described in above section.
Data analysis
The pharmacokinetic parameters for fuzapladib were calculated by means of noncompartmental methods using WinNonlin® software (Ver. 4.1, Pharsight Corporation, Mountain View, CA). For statistical comparisons, one-way analysis of variance (ANOVA) with pairwise comparison by Fisher’s least significant difference procedure was used. A P-value of less than 0.05 was considered significant for all analyses.
Pharmacokinetic behavior of fuzapladib
To clarify the possible species and gender differences, pharmacokinetic studies were carried out in rats, cats and dogs for intravenously- or subcutaneously-administered fuzapladib sodium monohydrate (2.0 mg /kg body weight). After the injection of fuzapladib sodium monohydrate solution, the plasma concentration of fuzapladib was determined by chromatographic analysis at various time points (Figure 1A & 1B). With respect to intravenous administration (Figure 1A), there were significant differences in clearance among the species tested, the CLtot values of which were calculated to be 687±24 (male) and 730±110 mL/h.kg (female) in rats, 74±11 (male) and 55±4.7 mL/h.kg (female) in cats, and 16±2 (male) and 19±2.9 mL/h.kg (female) in dogs, respectively (Table 1). Due to slow elimination in dogs, systemic exposure (AUC0–inf) of fuzapladib in male dogs was much higher than that in rats and cats by ca. 40- and 4-folds, respectively. Gender differences in pharmacokinetic behavior was negligible for rats and dogs, although there was a slight differences in pharmacokinetic behavior between male and female cats.
|
|
|
Cmax (mg/mL) |
Tmax (h) |
ke (1/h) |
AUC0–inf. (mg.h/mL) |
CLtot (mL/h.kg) |
|
Intravenous injection |
||||||
|
Rats |
Male |
– |
– |
1.9±0.13 |
3.3±0.17 |
687±24 |
|
Female |
– |
– |
1.3±0.11 |
3.2±0.70 |
730±110 |
|
|
Cats |
Male |
– |
– |
0.36±0.075 |
30±4.8 |
74±11 |
|
Female |
– |
– |
0.21±0.041 |
37±3.2 |
55±4.7 |
|
|
Dogs |
Male |
– |
– |
0.15±0.024 |
130±21 |
16±2.0 |
|
Female |
– |
– |
0.063±0.025 |
120±20 |
19±2.9 |
|
|
Subcutaneous injection |
||||||
|
Rats |
Male |
3.2±0.19 |
0.31±0.054 |
2.1±0.082 |
3.4±0.59 |
660±96 |
|
Female |
3.0±0.097 |
0.21±0.036 |
1.6±0.11 |
3.5±0.15 |
580±26 |
|
|
Cats |
Male |
6.6±0.53 |
0.88±0.11 |
0.30±0.064 |
22±1.7 |
93±8.2 |
|
Female |
10±1.2 |
0.63±0.11 |
0.38±0.043 |
35±4.4 |
61±7.7 |
|
|
Dogs |
Male |
15±0.34 |
0.44±0.054 |
0.13±0.022 |
110±4.9 |
18±0.83 |
|
Female |
13±0.36 |
0.88±0.11 |
0.13±0.020 |
91±8.7 |
23±2.5 |
Table 1 Pharmacokinetic parameters of fuzapladib sodium monohydrate (2.0 mg /kg body weight) in rats, cats, and dogs
Cmax: maximum concentration; Tmax: time to maximum concentration; ke: apparent elimination rate constant; AUC0–inf: area under the curve of blood concentration vs. time from t=0 to t=¥ after administration; and CLtot: total clearance. Values are expressed as means±SE from 4–5 experiments.
Similar outcomes were also observed for subcutaneously-administered fuzapladib sodium monohydrate (Figure 1B). After subcutaneous administration, fuzapladib was gradually absorbed with Tmax value of 0.2–0.9 h in all species, and fuzapladib decreased following first-order elimination kinetics. The slow absorption after subcutaneous administration may lead to an absorption-rate–limited pharmacokinetics and thereby, a prolonged systemic exposure as compared to intravenous administration,19 although drawbacks of subcutaneous administration may include incomplete bioavailability. Apparent elimination rate constants for male rats, cats and dogs were calculated to be 2.1, 0.3 and 0.13 h-1, respectively, and Cmax and AUC0–inf of male dogs were found to be ca. 5- and 32-fold higher than those of male rats, suggesting that clearance would be an important determinant of the bioavailability and of Cmax. Both rats and dogs exhibited almost identical pharmacokinetic behavior between male and female, and female cats tended to show slightly higher systemic exposure compared to males.
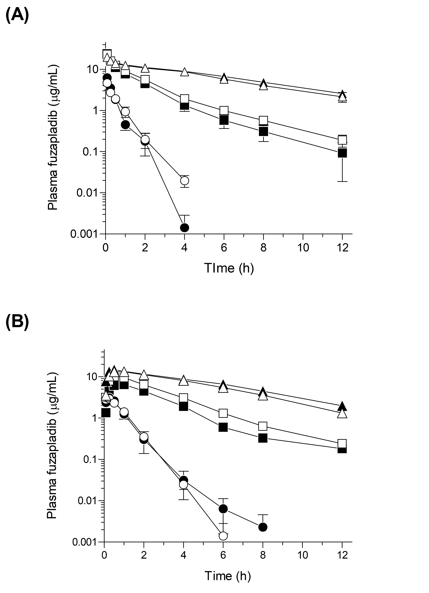
Figure 1 Pharmacokinetic behaviors of fuzapladib sodium monohydrate (2.0 mg/kg body weight) in rats, cats, and dogs following (A) intravenous and (B) subcutaneous injection. ●, male rats; ○, female rats; ■, male cats; □, female cats; ▲, male dogs; and △, female dogs. Data represent mean±SE of 4–5 experiments.
Pharmacokinetics of fuzapladib following both intravenous and subcutaneous administrations revealed significant inter-species differences; suggesting that the dose of fuzapladib for the treatment of acute pancreatitis might be variable among species. Although its precise mechanism is still unclear, there might be general differences in metabolic rate and pathways among species, leading to the variance in elimination kinetics.
Metabolic pathways of fuzapladib
Absorbed drugs can be distributed reversibly to various tissues of the body including the eliminating organs, such as liver and kidney, resulting in a decrease in blood drug concentration. Marked species differences in clearance of fuzapladib prompted us to perform preliminary studies on metabolism of fuzapladib in each animal species. In the present study, after incubation with liver S9 fractions of each species for 60 min, fuzapladib underwent enzymatic degradation, gradually; decreasing by ca. 38% for rat, 48% for cat, and 17% for dog liver S9 fractions, respectively (data not shown). To clarify the possible contribution of various CYP isoenzymes in the hepatic metabolism of fuzapladib, the potent inhibitors for CYP 1A (α-naphthoflavone), 3A (ketoconazole), 2B (ticlopidine), 2C (phenytoin), 2D (quinidine), and 2E (disulfiram) were added to reaction mixture and subjected to the chromatographic analyses. Some CYP inhibitors attenuated the enzymatic degradation of fuzapladib significantly (Figure 2). Significant inhibition on the enzymatic degradation was seen for CYP 2E (ca. 15%) and 3A (ca. 59%) inhibitors in the rat liver S9 fraction and for CYP 3A inhibitor (ca. 20%) in the cat liver S9 fraction. Interestingly, in the dog liver S9 fraction, all the CYP inhibitors tended to attenuate the degradation of fuzapladib, and, in particular, CYP 2B and 2C inhibitors achieved ca. 40% reduction in the degradation of fuzapladib. Although the contribution of other CYP isoenzymes remains unknown, metabolism of fuzapladib appears to be different among species.
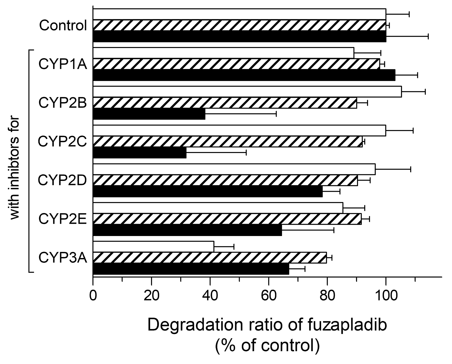
Figure 2 Metabolism of fuzapladib sodium monohydrate with or without CYP inhibitors. Fuzapladib sodium monohydrate was incubated for 60 min with S9 fractions from rats (open bars), cats (hatched bars), and dogs (filled bars) in the presence of inhibitors for CYP 1A (α-naphthoflavone), 3A (ketoconazole), 2B (ticlopidine), 2C (phenytoin), 2D (quinidine), and 2E (disulfiram). Data represent mean±SE of 3 experiments.
Inter-species differences in pharmacokinetic behavior of fuzapladib may lead to different dose requirements between species and understanding pharmacokinetic characteristics should inform the design of initial dose characterization studies. Additionally, due to the potential of hepatic dysfunction in animals suffering from acute pancreatitis, pharmacokinetic studies should be performed using a suitable acute pancreatitis model in order to determine whether clinically relevant differences exist between healthy animals and those with AP.
In the present study, inter-species differences between rats, cats and dogs were demonstrated in the pharmacokinetic behavior of fuzapladib sodium monohydrate after both intravenous and subcutaneous administration. Multiple CYP pathways were identified as important for metabolism, although not all potential CYP pathways were evaluated. Further biopharmaceutical characterization, taken together with the present findings, would be of help to define the dosing regimen of fuzapladib sodium monohydrate for other animal species.
This work was supported in part by JSPS KAKENHI [Grant-in-Aid for Scientific Research(C) (No. 20K07158; S. Onoue), and a Grant-in-Aid for Young Scientists (No.18K14885: H. Sato)].
The authors have no relevant financial interests that may influence the interpretation of our results.

©2022 Onoue, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.