eISSN: 2379-6367


Mini Review Volume 8 Issue 5
1School of Medicine, LABOATEM – Osteoarticular Biology, Tissue Engineering and Emerging Therapies Laboratory, Biological Chemistry Cat., School of Medicine, Rosario National University, Argentina
2Research Council of theRosario National University, (CIUNR) and CONICET, Argentina
Correspondence: Ivan Nadir Camal Ruggieri, LABOATEM, Santa Fe 3100, Rosario, Santa Fe, Argentina, Tel +5493416507995
Received: September 16, 2020 | Published: October 2, 2020
Citation: Ruggieri INC, Feldman S. Signaling pathways of nucleic acids for bone healing: A review. Pharm Pharmacol Int J. 2020;8(5):291-295. DOI: 10.15406/ppij.2020.08.00308
Different kinds of nucleic acid (NA) molecules are promising therapeutic tools in a variety of tissues and their pathogenesis, and bone pathologies are not the exception. NAs interact in cytosol or nucleus to generate a specific response. Some of these NAs can be used to generate a positive response in relation to bone formation and differentiation in osteoblast and osteocyte. This work aims to briefly and clearly show main signaling pathways in osteoblasts and osteocytes, and to state the mechanism of how miRNA agonists (miRNA) and miRNA antagonists (antagomir) affect them. Thus, this summarizes the mechanism of promising therapeutic strategies for bone repair. NAs are fragile and can be degraded quickly outside the cells. These problems are more and more frequently resolved by nanotechnology and tissue engineering approaches. Further research in this field wills probably generate safe and therapeutic effective therapy in relation to bone healing.
Keywords: bone repair, miRNA, antagomir, osterix, runx2, signaling pathway, wnt
NA, nucleic acid; siRNA, small interfering RNA; miRNA, microRNA analog; antagomir, microRNA antagonist; mRNA, messenger RNA; Fz, Frizzled; LRP5/6, low-density lipoprotein receptor-related protein 5 or 6; ROR, RAR-related orphan receptor; APC, activated protein C; GSK3, glycogen synthase kinase 3; CK1, casein kinase; TCF/LEF, T cell factor/lymphoid enhancer factor 1; OCN, osteocalcin; OPN, osteopontin; RANK-L, receptor activator for nuclear factor κB ligand; ALP, alkaline phosphatase; OPG, osteoprotegerin; Col1, type 1 collagen; MMP-13, matrix metallopeptidase 13; PCP, planar cell polarity; YAP-TAZ, yes-associated protein – transcriptional co-activator with PDZ-binding motif; Wnt3a, Wnt family member 3a; DKK-1, dikkopf-related protein 1; SFRP-1, secreted Frizzled-related protein 1; Wnt5a, Wnt family member 5a; Wnt5b, Wnt family member 5b; BMP, bone morphogenetic protein; BMPr1, BMP receptor 1; BMPr2, BMP receptor 2; R-Smads, receptor-regulated Smads; I-Smads, inhibitory Smads; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun amino-terminal kinase; mTOR, mammalian target of rapamycin; Bcl2, B-cell lymphoma 2; Mitf, microphthalmia-associated transcription factor; Bim or Bcl2L11, Bcl2-like protein 11
In the last decades nucleic acids (NA) are studied as therapeutic tools thanks to interacting at the genetic level, and then generating a specific response. In order to uncover their molecular mechanism, it is important to understand the signaling pathways of the different cellular populations. This work aims to state a clear view of promising NAs and their molecular mechanism in relation to bone-targeted therapy.
Therapeutical NAs can be separated into two groups. One group is formed by plasmid DNA, small interfering RNAs (siRNA), ribozymes, deoxyribozymes, microRNA analogs (miRNA), and microRNA inhibitors (antagomir); which are base pairing complementary to the target. The other group is formed by aptamers, messenger RNAs (mRNA), and CpG; which are not base pairing complementary to the target. NAs are very fragile biomolecules outside the nucleus environment.1Thus, NAs are easily destroyed either in blood, by renal clearance, or hydroxylation by ribozymes and deoxyribozymes. This is why NAs need a special mechanism to be delivered into the cells. Nanoscale biotechnology and tissue engineering have been remarkably important to aim this goal. There is a great variety of nanocarriers for therapeutic NAs that are in preclinical phases and others are already in clinical ones.2-7
Understanding the complicated and intricated signaling pathways is a key requirement in order to correctly apply future strategies with NAs. In relation to bone, there is a vast bibliography that compiles the main mechanisms that regulate the balance of bone turnover. In this paper, we present in a summarized way and, trying to be simple, the main metabolic pathways and their responses in relation to cellular differentiation and bone formation.
Wnt pathways
Wnts are a superfamily of ligands, formed by a chain from 340 to 380 amino acids. Wnts proteins bind to its receptor, a Frizzled protein (Fz) joined to a low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6). Four kinds of signaling pathways assigned to Wnt-ligands have been discovered. One of these signaling pathways is commonly known as the canonical way, while the others are named “non-canonical” ways. This regards the timing of the mechanism chronologically discovered. The non-canonical ways are divided in three trunks; two are activated by the same receptor as the canonical way, but one is activated by a receptor made of Fz joined to RAR-related orphan receptor (ROR).8,9
The canonical way depends on β-catenin. In normal conditions, these proteins join between them, and rapidly, they are destroyed by an APC-Axin-GSK3-CK1 formed complex. This maintains a low concentration of intracellular β-catenin union. The mechanism is triggered due GSK3, which phosphorylates β-catenins. Phosphorylated β-catenins are ubiquitinated and then degraded by proteasome. When Wnts ligands bind toFz and LRP5/6, APC-Axin-GSK3-CK1 complex is inhibited, resulting in β-catenins accumulation in cytosol, and then formation of β-catenins complex, that triggers translocation to the nucleus. β-catenins complexes regulate specific T cell factor/lymphoid enhancer factor (TCF/LEF). TCF/LEF regulates Runx2 and Sp7/Osterix, both are the most described and analyzed transcription factors involved in bone formation and differentiation. Both Runx2 and Sp7/Osterix, positively regulates mRNA of proteins like osteocalcin (OCN), osteopontin (OPN), RANK-L, ALP, OPG, Col1, MMP-13, and bone sialoprotein.10-13
Wnts are a family ligand. On one hand, the role model of agonism of Wnt family members is Wnt3a. But in another, there is a group of Wnt antagonists like DKK-1, sclerostin, SFRP-1, Wnt5a, and Wnt5b, among others. These proteins are full antagonists of Fz and LRP5/6 receptors. The binding of these proteins to Fz-LRP5/6 regulate by negative feedback on the Wnt pathway.14-17 The non-canonical ways like Ca++ pathway, PCP pathway and YAP-TAZ pathway are also fundamental for bone formation and differentiation, but we will not discuss them here because the different NAs described in this work interact with participants of the canonical way. We invited to interested and curious readers to expand the reading with the alternative Wnts ways to have a widely panorama of this complex signaling pathways.18-22
BMP
Bone morphogenetic protein (BMP) is growth factors and members of the transforming growth factor β (TGF-β). BMP binds to a receptor, that is confirmed by BMP receptor 1 (BMPr1) alone or together with BMP receptor 2 (BMPr2). When BMPs bind to the receptor, this triggers the activation of Smad1, Smad5, and Smad8. These three Smad proteins are known as receptor-regulated Smads (R-Smads). R-Smads binds to Smad4, and then translocase to the nucleus to stimulate Runx2. Smad6 and Smad7, another Smad proteins, inhibit R-Smads complex, and are known as inhibitory Smads (I-Smads).8,23,24
ERK
Mitogen-activated protein kinase (MAPK) controls a great amount of physiological processes. MAPKs activate other kinases, like extracellular signal-regulated kinase (ERK), or c-Jun amino-terminal kinase (JNK). Particularly, ERK activates critical transcription factors like Runx2, and Sp7/Osterix through Sp1, but also E2F, mTOR. mTOR activates Bcl2, and Mitf. Mitf inhibits Bim. This actions triggered by mTOR result in decreased apoptosis and increased survival.25-27
In Figure 1, we briefly summarized the main mediators of Wnt, BMP, and ERK signaling pathways, and their principal transcription factors.
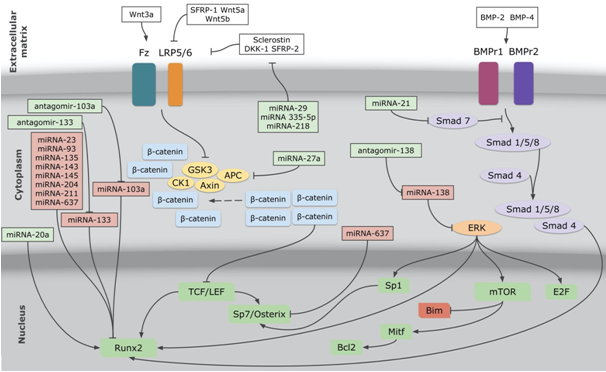
Figure 1 The figure shows miRNAs and antagomirs, and their interaction with Wnt, BMP and ERK signaling pathways. Connectors with arrows mean stimulation, connectors with flat ending mean inhibition. miRNAs and antagomirs in green boxes mean that they have, as finally response, stimulation of bone formation and differentiation. miRNAs in red boxes mean that they have, as finally response, inhibition of bone formation and differentiation. Transcription factors in green boxes mean that they have, as finally response, stimulation of bone formation and differentiation. Bim in the red box mean that it has, as finally response, inhibition of bone formation. miRNA, microRNA analog; antagomir, microRNA antagonist; Fz, Frizzled; LRP5/6, low-density lipoprotein receptor-related protein 5 or 6; APC, activated protein C; GSK3, glycogen synthase kinase 3; CK1, casein kinase ; TCF/LEF, T cell factor/lymphoid enhancer factor 1; Wnt3a, Wnt family member 3a; DKK-1, dikkopf-related protein 1; SFRP-1, secreted Frizzled-related protein 1;SFRP-2, secreted Frizzled-relatedprotein2; Wnt5a, Wnt family member 5a; Wnt5b, Wnt family member 5b; BMP-2, bone morphogenetic protein 2; BMP-4, bone morphogenetic protein 4; BMPr1, bone morphogenetic protein receptor 1; BMPr2, bone morphogenetic protein receptor 2; BMPr1, BMP receptor 1; BMPr2, BMP receptor 2;ERK, extracellular signal-regulated kinase; mTOR, mammalian target of rapamycin; Bcl2, B-cell lymphoma 2; Mitf, microphthalmia-associated transcription factor; Bim or Bcl2L11, Bcl2-like protein 11.
There are four types of NAs, siRNAs conjugated to N-acetylgalactosamine (GalNac) more specifically, that are in phase III of clinical trials. These drugs are lumasiran, vutrisiran, givosiran, and inclisiran, which are agents against primary hyperoxaluria type 1, transthyretin mediated amyloidosis, acute hepatic porphyria, and hypercholesterolemia, respectively.1,28-31
Like the mentioned siRNAs, there are a huge group of miRNAs and antagomirs that are already identified as remarkably stimulators of osteoblast/osteocytic lineage differentiation, proliferation, and survival. In Table 1, we show the most studied miRNAs and antagomirs in relation to bone signaling pathways, their targets, and their final response to bone turnover. In Figure 1, we show how these NAs interact with aforementioned main signaling pathways.
miRNAs and antagomirs (* = in vitro and/or in vivo studies) |
Targets (underline = target stimulated; italics = target inhibited) |
Stimulate bone formation and differentiation |
Inhibit bone formation and differentiation |
miRNA-20a* |
Runx2 |
X |
|
miRNA-21* |
Smad7 |
X |
|
miRNA-23a |
Runx2 |
X |
|
miRNA-27a |
APC |
X |
|
miRNA-29a |
Sclerostin, DKK-1, SFRP-2 |
X |
|
miRNA-93 |
Runx2 |
X |
|
miRNA-103 |
Runx2 |
X |
|
miRNA-133a* |
Runx2 |
X |
|
miRNA-135 |
Runx2 |
X |
|
miRNA-143 |
Runx2 |
X |
|
miRNA-145 |
Runx2 |
X |
|
miRNA-138 |
ERK |
X |
|
miRNA-204 |
Runx2 |
X |
|
miRNA-211 |
Runx2 |
X |
|
miRNA-218 |
Sclerostin, DKK-1, SFRP-2 |
X |
|
miRNA-335-5p* |
Sclerostin, DKK-1, SFRP-2 |
X |
|
miRNA-637 |
Runx2, Sp7/Osterix |
X |
|
antagomir-103a |
miRNA-103a |
X |
|
antagomir-133a* |
miRNA-133a |
X |
|
antagomir-138* |
miRNA-138 |
X |
|
Table 1 List of microRNA analogs (miRNA) and microRNA antagonists (antagomir), their targets, and their final response in bone turnover miRNA, microRNA analog; antagomir, microRNA antagonist; APC, activated protein C; DKK-1, dikkopf-related protein 1; SFRP-2, secreted Frizzled-related protein2; ERK, extracellular signal-regulated kinase.
As we already mentioned, nucleic acid-based therapy has a big amount of challenges. NAs are rapidly degraded by endonucleases. Nucleases are key to protecting us from virus invasion and missense RNA or DNA accumulation. Further, NA molecules have a very rapid renal clearance in blood. It is shown that the half-life of plasmid DNA following IV infusion is closely to 10 minutes. Besides, NAs are negatively charged molecules, so they cannot diffuse across cell membranes.32-35 These challenges are being more and more overcome thanks to nanoscale technology. Vectors consist of entrapping NA molecules in suitable nanocarriers with the objective of avoiding the aforementioned barriers.36,37 Then are the latest strategies of different vectors for miRNAs and antagomirs against bone diseases used in vitro and/or in vivo studies.
Castaño and cols. al. used collagen-nanohydroxyapatite scaffolds with miRNA-133a, delivered with non-viral particles. They showed that this scaffold enhanced human mesenchymal stem cells through activation of Runx2.38 Nguyen and cols. used polyethylene glycol hydrogels to create a scaffold, with miRNA. They demonstrated that the delivery of miRNA-20a from the hydrogel constructs enhanced the osteogenic differentiation.39 Eskildsen and cols. demonstrate that antagomir-138 regulates osteogenic differentiation of human mesenchymal stem cells in vivo40, and Wu and cols. used hydrogels with antagomir-138, demonstrating a significantly enhanced bone regeneration compared to blank group, as long as increased levels of OCN, OPN, and Col1.41 Sui and cols. used nanomaterials to deliver miRNA-355-5p using a lipidoid formulation into osteogenic cells for tissue engineering applications. In that paper and in previous studies, this group concluded that miRNA-355-5p interacts with Wnt signaling pathways in regulating bone development and homeostasis.42,43 Wang and cols. used a microarc-oxidized titanium surface with miRNA-21-loaded hyaluronic acid nanoparticles, and they confirmed that this scaffold promotes osteogenic differentiation on human bone marrow mesenchymal stem cells.44-46
There are no clinical trials entries using miRNAs or antagomirs as bone diseases therapy in clinicaltrial.gov to date. However, there is study from Moscow State University started in 2017, that evaluates the safety and efficacy of a gene-activated bone substitute consisting of octacalcium phosphate and plasmid DNA encoding vascular endothelial growth factor for maxillofacial bone regeneration. In this clinical trial were enrolled 20 participants with either congenital or acquired maxillofacial bone defects or alveolar ridge atrophy. All participants received treatment. The safety and efficacy of the implanted bone substitute was evaluated by clinical examination, comprehensive laboratory test, and computer tomography within 6 months after surgery. There is no results yet.47-49
In the latest decades, the number of researches in relation with miRNA, antagomirs increased significantly. Not only as therapeutics, but also as diagnostic biomarkers. miRNA expression patterns specific types of bone diseases, and this can be used as diagnostic tools. Kocijan and cols. identified significant differences in serum levels of circulating miRNAs both in premenopausal women and posmenopausal, even in men. They identified a group of 19 miRNAs that were excellent discriminators of low-traumatic fractures, regardless of age and gender.50 Like Kocijan and cols., there are multiple groups studying the changes of different miRNAs levels in serum and/or plasma around the globe, involving different types of population. miRNA-21, miRNA-23a, miRNA-133a, among others miRNAs circulating in serum and/or plasma have a direct correlation with bone mineral density, independent of gender in osteoporotic patients.51-55 The European Company TAmiRNAGmbH developed osteomiR®,first osteoporotic and high fracture risk diagnostic test based on miRNA in serum or plasma. OsteomiR®- uses qPCR amplification of different types of miRNAs.56
Circulating levels of miRNAs were also studied during osteoporosis treatment. Teriparatide and denosumab are two very potent anti-osteoporotic medications. Teriparatide mimics human parathyroid hormone, promoting calcium absorption and bone formation. Denosumab is a monoclonal antibody against RANKL, preventing RANK activation and osteoclast formation. Both drugs were studied in relation to changes in miRNAs expression during treatment.57,58 miRNA-133a and miRNA-33 levels were significantly decreased after treatment with teriparatide.51,52,59 In relation with denosumab, no significant change of miRNAs levels was observed during treatment.58,60
The knowledge of main signaling pathways is of utmost relevance to understanding the different mechanisms of miRNAs and antagomirs. Noting the difficulties of these molecules in relation to pharmacokinetic is important to generate specific responses using nanotechnology and tissue engineering techniques. Beyond those already mentioned and well-documented in vitro and/or in vivo studies, we are still far from secure, safe, and effective miRNAs and/or antagomirs-scaffolds for extended use at the clinical level for bone healing in different pathologies. Research into new miRNAs and antagomirs, new delivery strategies and other methods to overcome NAs structural difficulties, and progress towards controlled-clinical studies are key to advancing on bone healing.
None.
Authors declare that there is no conflict of interest.

©2020 Ruggieri, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.