eISSN: 2379-6367


Research Article Volume 9 Issue 2
1Immunology reproduction laboratory (LIR), Argentina
2Biochemistry and Pharmacy School, National University of Rosario (UNR), Argentina
3Research council from National University of Rosario (CIUNR), Argentina
4University of Morón (UM), Argentina
Correspondence: Raspo Esteban, University of Morón (UM), 24 de Septiembre 1125, Rosario, Santa Fe, Argentina, Tel +5493415080076
Received: February 26, 2021 | Published: April 9, 2021
Citation: Raspo E, Brunori M, Brufman A. Seminal transferrin, a potential human testis biomarker. Pharm Pharmacol Int J. 2021;9(2):51-56. DOI: 10.15406/ppij.2021.09.00327
Introduction: Seminal plasma (SP) is a sperm function modulator, for this reason, it is necessary to adequately characterize its molecular composition. Iron (Ir) plays an important role in spermatogenesis. Transferrin (Tf), the cellular membrane receptor for transferrin and ferritin (FN), respectively, are involved in the transport, cellular uptake, and storage of Ir. The metabolic pathways of Ir are still unknown in human testis.
Objectives: The aim of the work was to study biochemical components such as total proteins (TP), Ir, FN and Tf in SP of young individuals, and to establish reference values for our laboratory. These values were then correlated to semen parameters and some sperm functional tests in order to understand the physiology of Ir in the testis.
Materials and methods: Two hundred and fifteen semen and serum samples were studied, and the concentration of Tf, Ir, TP and FN was determined in both fluids. A basic semen analysis and sperm vitality tests were performed. Statistical analysis by the Spearman coefficient (r) for testicular transferrin (TfT) versus each of the quantitative variables of the basic semen analysis was used.
Results and conclusions: It was observed that TfT concentration has a direct relationship with the sperm concentration (r=0.3872, p-value=0.0070, p<0.01) the total sperm count (r=0.515; p-value=0.0008, p<0.01) and to a lesser extent with the percentage of progressive motile sperm (r=0.3721; p-value=0.0139, p<0.05). No correlation with morphology was found. The study of possible biomarkers, such as TfT, would contribute to the knowledge of physiological mechanisms of sperm function, enabling an accurate diagnosis and proper treatment.
Keywords: seminal plasma, transferrin, sperm cell, human infertility, biomarker, human testis
SP, seminal plasma; Tf, transferrin; RTf, membrane receptor for transferrin; Ir, iron; FN, ferritin; TfT, testicular transferrin; CS, Sertoli cells; RB, reasidual body; CNS, central nervous system; TP, total protein; WHO, world health organization; RID, radial immunodiffusion; LRL, lower Reference Limit; M, mean; SD, standard deviation; CV, coefficient of variation; SFT, seminiferous tubules; SG, spermatogonia
Infertility affects 15% of couples worldwide. In half of the cases it is related to the male factor.1 Semen analysis is the initial step in the study of the male factor. In many cases, the cause of infertility is not identified and is classified as idiopathic, which suggests little knowledge about the mechanisms that regulates spermatogenesis and sperm function.2 In these cases, routine semen analysis does not explain fertilization abnormalities,3 perhaps due to insufficient knowledge of the basic mechanisms of the reproductive process.4
A proper diagnosis of male infertility is of great importance to assess whether or not an abnormality will be passed on to the embryos. Furthermore, there is a necessity to develop better seminal diagnostic biomarkers to assess the success rates of assisted reproductive technologies. A greater understanding in the field of epigenetics is much needed to improve diagnosis and thus develop more personalized treatments for men with idiopathic infertility.5
Semen is divided into two main components: the cellular one, which represents 10% of the total ejaculate volume, and the acellular, generically called seminal plasma (SP).6 This fluid contains various components, including lipids, carbohydrates, ions, peptides, and proteins, which are secreted by different regions of the male reproductive tract. It does not only act as a mean of transport, protection and nutrition of sperm, but also as a modulator of sperm function. For this reason, it is necessary to properly characterize the molecular composition of SP.
Seminal plasma proteins perform a wide variety of functions: protection by binding to the sperm surface during ejaculation and playing a key role in the capacitation, acrosome reaction, and fusion of sperm and egg. Because of this specificity it is considered that seminal plasma proteins can serve as important biomarkers for male infertility.7 The protein profile of SP is a growing topic, which is greatly enriched by the combination of proteomic analyzes and functional studies which, together, will allow us to discover new cellular pathways that will help provide a better understanding male infertility. The ability to predict fertility using biomarkers is a promising field.
To date, numerous seminal proteins which could be considered important biomarkers for male infertility have been documented, but no noticeable progress has been made in the field.8 Iron plays an important role in spermatogenesis. This nutrient is essential but, in excess, it can cause toxicity. Iron transport and its absorption systems are strictly regulated in all organisms dependent on this element. Iron transport, cell uptake, and storage involve Tf, the cell membrane receptor for transferrin (RTf), and ferritin (FN), respectively. Although at the systemic level the metabolic pathways of iron are established, the mechanisms are still unknown in the testis.
Serum Tf is synthesized mainly by hepatocytes at a concentration of 2.5 mg/ml and 70% is found as apotransferrin, not bound to Ir.9 It can also be synthesized in cells of the mammary gland, testis, central nervous system (CNS), lymphocytes, and macrophages. 1.5% of the total proteins in seminal plasma is constituted by an isoform of Tf, called testicular transferrin (TfT), which is secreted by Sertoli cells (CS).10 Griswold in 1984,11 postulated that TfT was involved in the delivery of Ir in developing germ cells. Brotherton and his colleagues12 studied variations in seminal Tf results in 1990 with six different commercial kits in 43 apparently healthy individuals and found a very low correlation. With the same objective, Ford13 measured serum transferrin levels in 60 chronic kidney patients with the six equipment most commonly used in clinical practice and found a difference in the results of up to 150ng/mL. In the same study, a biological variation that ranged from 8 to 18% was found. FN is an iron-binding molecule, whose main function is to store this element to guarantee its availability in different cellular functions. It also plays an important role in inflammation, neurodegenerative diseases, and cancer (Figure 1).
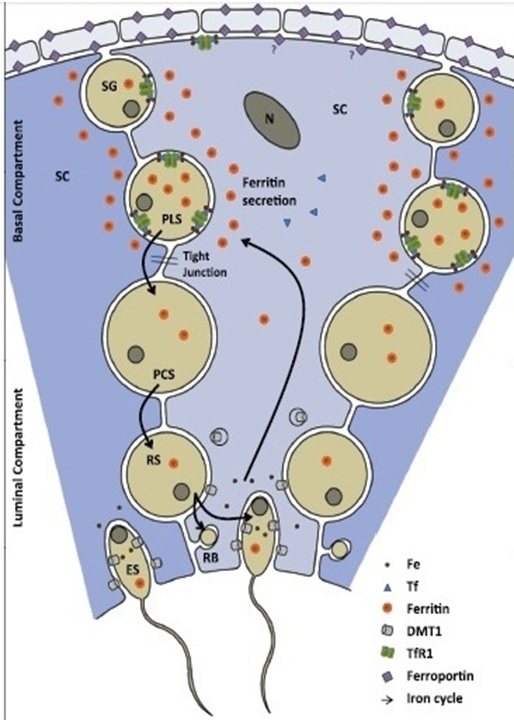
Figure 1 Shows a model of iron transport in murine testis.14
Three CS and their immediate surroundings are shown. Iron transport across the seminiferous tubules (SFT) basal membrane is very limited. Within the SFT some spermatogonia (SG) and mainly primary spermatocytes acquire iron-loaded FN from CS, and upon maturation elongating spermatids return iron to the CS, which traffic it back to a new generation of spermatocytes. Obligatory iron losses through spermatozoa that leave the testis are replenished by the peripheral circulation through the transferrin-TfR1 system. Ferroportin likely plays its main role in iron trafficking across the interstitial space, where selective barriers at the smooth muscle cells of blood vessels and the peritubular myoid cells provide the male germ cells with additional protection from the periphery RB, residual body.14
RTf expression is regulated (negatively) at transcriptional level by iron concentration. Thus, with low iron levels, half-life of the translated mRNA is prolonged and the receptor density on the cell membrane increases. As a consequence, Tf uptake by the cell increases and iron requirements are ultimately satisfied. When cellular iron levels are high, the opposite occurs.
Objective
The aim of the work was to study biochemical components such as TP, Ir, FN and Tf in SP of young individuals, and to establish reference values for our laboratory. These values were then correlated to semen parameters and some sperm functional tests in order to understand the physiology of iron in the testis.
Two hundred and fifteen semen and serum samples from patients from the Urology Service of “Eva Perón” Training Hospital (Granadero Baigorria), Centenario Hospital (Rosario) and healthy volunteers (age: 36.55±10.44) were studied from November 2018 to September 2019. Samples were obtained with the prior informed consent of the patients and controls with the authorization and endorsement of the treating professionals according to Helsinki´s declaration. The framework project for this study has the approval of the bioethics committee from the School of Biochemical and Pharmaceutical Sciences, Exp. 6060/368. Res. CD 592/2017. Patients received written instructions for sample collection. Semen samples were collected by masturbation with 48/72 h of abstinence. Seminal samples with low volume (<1ml) and patients with clinical conditions that could interfere with the levels of Tf and FN in blood plasma such as acute or chronic liver disease, neoplasia, clinical / laboratory signs of infection / acute inflammation or chronic hepatitis virus (A, B or C) infections, leukocytosis, fever, hypoproteinemia and iron metabolism diseases were not included.
Processing of semen samples
After the liquefaction of semen samples (30-60 min after collection), a complete semen analysis was performed following WHO 2010 guidelines,15 which include macroscopic and microscopic examination. Macroscopic examination consists of: volume, appearance, consistency and pH. After examination, two sample aliquots were separated, 300µL for microscopic investigation, and the rest of the semen sample was centrifuged for 30 minutes at 5000rpm to obtain seminal plasma, which was kept refrigerated (-20°C) for subsequent analysis. In light microscopic (Leica DM500) was evaluated: motility on a thermostated stage with a magnification of 400x, agglutination, and vitality with supravital eosin staining. Sperm cell concentration was performed with an improved Neubauer´s chamber. Hematoxylin-eosin staining was used to evaluate morphology with immersion lens (1000X).
Preparation of serum samples
10mL blood collection without anticoagulant was carried out after venipuncture of the upper limb. After exudation (approximately 30 minutes in a water bath at 37°C), blood was centrifuged at 3,500 rpm for 10 minutes. Serum was stored at -20ºC until laboratory determinations were made.
Biochemical determinations
To determinate quantifications, Cobas C501® analyzer (Roche Diagnostics) was used, measuring the seminal plasma and serum of the patients under study. All reagents and controls were from Roche-Diagnostics®:
Iron quantification: Colorimetric method without deproteinization with Ferrozime (reading at 600nm).Total protein content was assessed using the modified Lowry bicinchoninic acid (BCA) assay, in which amino acid residues from proteins react with copper ions and bicinchoninic acid to produce a color shift which is linear in response to protein concentration. Thus, contaminant sugars, lipids and metabolites will not interfere with protein quantification.16 Ferritin evaluation was carried out with Elecsys® Ferritin-Roche electrochemiluminescence immunoassay.
Radial immunodiffusion (RID) technique was used to measure Tf concentration in both fluids. In serum, it was quantified with commercial RID plate DIFFU-PLATE® (Biocientifica SA), to measure Tf in SP, a RID adapted (to low concentrations) plate was developed in our laboratory. For RID plate construction, agarose (Fisher Scientific) 1.5 P/V in Veronal Sodium-Veronal buffer (0.02 M with pH 8.6 and 0.005 M sodium chloride) added an specific antibody for Tf (Polyclonal generated in rabbit, Code: OSAX, Dade Behring Inc. Newark, USA) to reach a final concentration of 3.80±1.30 mg/dL (mean±SD).
Sperm vitality test-supravital stain
Sperm cell vitality estimates the membrane integrity of cells and it’s especially important when progressive motile sperm count is less than 40%.15 To perform supravital staining, 20μL of homogenized whole semen were placed on a slide and 50μL of eosinY (E4009-Sigma Aldrich) ([(0.5% (w/v) in NaCl 0.9% (w/v)]. It was observed under a light microscope (Leica DM 500) with a magnification of 400x. Living cells do not incorporate the stain and a white or light pink color is observed while dead cells have a reddish coloration on their heads. Lower Reference Limit (LRL)15 for vitality is 58%.
Confirmatory proteomics analysis–western blotting
Sample preparation
An alicuot of 100μl of serum an seminal plasma were supplemented with phenylmethylsulfonyl fluoride(PMSF), then, a centrifugation was carried out at 14000 g at 4ºC for 15 min. Tf and TfT were selected for confirmatory proteomics analysis by one-dimensional gel electrophoresis (1DGE) followed by western blotting. Briefly, 20μg of seminal plasma and serum proteins were suspended in Milli-Q water, which was diluted 1:1 (v:v) in sample buffer [0.125 M Tris-HCl, pH 6.8, 4% (w:v) SDS, 20% (v:v) glycerol, 5% (v:v) beta-mercaptoethanol,0.02% bromophenol blue] and boiled at 100 °C for5 min. Proteins were then separated in 10% polyacrylamide gels under denaturing conditions, and transferred to nitrocellulose membranes through a Wet transfer system (MiniVE; GE Healthcare, Amersham Place, UK). Membranes were subsequently incubated in blocking buffer [3% bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween-20 (TTBS)] for 1 h, washed in TTBS, and incubate with primary antibodies against Tranferrin (sc-21011, Santa Cruz Biotechnology Inc.Santa Cruz , USA) for 2 h at room temperature. Membranes were then washed with TTBS and incubated with horseradish peroxidase (HRP)-conjugated appropriate secondary antibodies for 1 h at room temperature. Detection was performed using enhanced chemiluminescence (ECL; GE Healthcare). Signals were detected using an Image- Quant LAS 4000 system (GE Healthcare).
Statistical analysis
Due to the lack of normality in the distribution between variables the Spearman coefficient was used to verify a possible correlation. For Tf concentrations comparison a categorization into groups was carried out, the non-parametric Mann Whitney test was applied due to non-compliance with the assumption of normality. R Commander® and Excel (Microsoft Co) programs were used for database. Statistical significance was considered when p <0.05.
Patient´s values
S and SP samples were quantified for TP, Tf, Ir and FN according to the methods described above. The results obtained were the following. Table 1 shows obtained values: mean (M), standard deviation (SD) and coefficient of variation (CV) for TP, FN, Ir, Tf in SP. Table 2 shows obtained values: mean (M), standard deviation (SD) and coefficient of variation (CV) for TP, FN, Ir, Tf in serum (Figure 2-3-4).
|
Variables in SP |
Minimum |
Maximun |
Mean (M) |
Standard |
M±SD |
Median |
Coefficient of |
|
Protein (g/dL) |
0,48 |
5,5 |
2,3 |
0,97 |
2,3±0,97 |
2,18 |
42,2 |
|
Ferritin (ng/dL) |
20 |
556,3 |
263,1 |
111,12 |
263,1±111,12 |
272 |
42,2 |
|
Iron (µg/dL) |
12 |
71 |
30,7 |
13,79 |
30,7±13,79 |
28,5 |
44,9 |
|
Transferrin (mg/dL) |
1,58 |
16,33 |
4,71 |
3,64 |
4,71±3,64 |
3,35 |
76,9 |
Table 1 Metabolites in SP
|
Variables in serum |
Minimum |
Maximun |
Mean (M) |
Standard |
M±SD |
Median |
Coefficient of |
|
Protein (g/dL) |
4,31 |
8,47 |
7,17 |
0,74 |
7,17±0,74 |
7,24 |
10,3 |
|
Ferritin (ng/dL) |
17,2 |
860 |
227,2 |
170,8 |
227,2±170,8 |
185,5 |
75,1 |
|
Iron (µg/dL) |
50 |
186 |
104,1 |
30,7 |
104,1±30,7 |
102 |
29,4 |
|
Transferrin (mg/dL) |
81,6 |
546,1 |
247,7 |
80,0 |
247,7±80 |
210,2 |
32,2 |
Table 2 Metabolites in serum
Analysis of variables
Different samples were selected considering different parameters established for them:
Iron/Transferrin
Those samples that contained low iron concentration were grouped in order to compare them with transferrin values in SP. It was observed that 82% of the samples with low iron concentration (≥30ug/dL) did not reach the population mean of Transferrin (5 mg/dL). Indicating there is a correlation between low iron concentrations and transferrin concentrations below 5 mg/dL.
Iron/Transferrin/Vitality
To study the relationship between low concentrations of iron and transferrin, compared to sperm cell vitality, we discarded those samples that did not comply with the above described. It was observed that there is no significant correlation between sperm vitality and these variables studied since only 17% of the samples showed decreased iron and transferrin concentration and low vitality.
Relative concentration/Transferrin
Relative concentration of sperm cells was studied compared to transferrin concentration. According to the WHO, LRL is 15 million cells per mL. This study was highly significant since none of the selected samples, which did not comply with the LRL, reached the population mean of Transferrin.
Progressive mobility/ Transferrin
Samples with progressive motile spermatozoa below the LRL (32%) were selected, correlating them to the concentration of transferrin. When comparing the percentage of progressive mobility with the concentration of transferrin in SP, we could observe that 85% did not reach the population mean.
To achieve a better visualization of diffusion halos, plates were stained with Amido Black (BioSystems) for 5 min and washed with decolorizing solution [acetic acid 25% (v/v); 40% methanol (v/v)]. Reading was made with a millimeter magnifying glass (0.1 mm precision) with a 10x magnification. Tf concentration is calculated using the following calibration curve: D2=0.6702x[Tf]+1.41651, R2=0.9839. Where [Tf] is the concentration of Tf in mg/dL and D2 is the squared diameter of the diffusion halo (Figure 5).
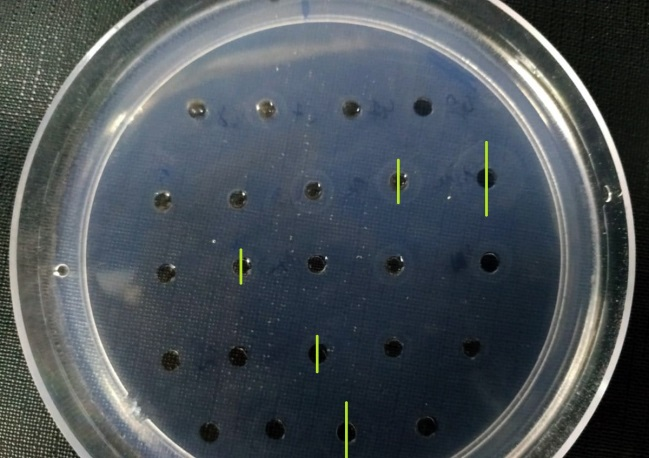
Figure 5 Transferrin concentration in SP. Samples measurement in laboratory designed plate. Example of halo diameters created by different samples according to its Tf concentration gel not stained.
Example of Immunodetection of Tf using anti-Tf antibody. Serum sample was dilute 1/100 and SP sample was dilute 1/2, both samples from the same patient. Lane 1: human serum (dilution 1/100), Lane 2: Negative control. Lane 3: Seminal plasma (dilution ½). Bands below correspond to loading control staining with Ponceau S. Under the assumption that murine TfT has been evidenced as responsible for iron transport in SFT we set out to corroborate it by immunodetection with the Western Blot technique (Figure 6).
Male infertility is a widespread medical condition with great social and emotional costs, and male factor is related in no less than 50% of these cases. The etiology of male infertility is multifactorial and often unknown. Basic semen analysis is the cornerstone in evaluating male factor infertility. However, this routine test does not provide information regarding sperm cell development and function. For this reason, it is necessary to identify biomarkers in seminal plasma to acquire more information about the metabolic and molecular processes of the male gamete.
In previous studies, very different values of metabolites Ir, Tf, FN and TP in SP were observed, for this reason it is necessary to have our own calculated reference values from our population and methodology to deepen in the knowledge of the homeostasis of the iron in testis. In our work, the studied biochemical markers were significantly different in seminal plasma and serum, and no relationship was found between them. These differences may be associated with the selective secretion of the testis, epididymis and male accessory glands and, also, the specific environment required for metabolism and maintenance of sperm function. In order to evaluate Tf as a possible biomarker, we related its concentration in PS in a heterogeneous group (composed of individuals with some andrological pathology and likely healthy controls), with quantitative variables of the basic semen analysis of greater clinical relevance.
According to Fuse,17 SP transferrin concentrations were decreased in hypergonadotropic oligozoospermic subjects and in men with azoospermia due to germ cell injury. The positive correlation, demonstrated in previous studies, between TfT concentration and sperm concentration and the decreased levels of this protein in patients with oligozoospermia confirms this.18 Skinner and Griswold19 assumed that Sertoli cells (SC) serve as intermediaries in the transport of iron from serum transferrin to developing germ cells. It was also shown that the growth promoting effect of this protein can be explained by its role in the supply of iron to testicular cells. Transferrin was further reported to bind to spermatocytes with high affinity. These findings indicate, once again, that transferrin plays an important role in the development and differentiation of spermatogenic cells.
As stated by Barthelemy,20 transferrin concentrations in vasectomized patients were significantly lower (approximately 5 times) than in normozoospermic men or proven fertile donors. This difference suggests that the main source of seminal fluid transferrin is testicular, as previously suggested.21 The remainder of the transferrin can originate from secretion of the prostate and/or seminal vesicles and/or serum transudation. However, the lack of a correlation between blood and seminal transferrin suggests low participation of serum component.21 In our results, we also found no relationship between these variables.
All the analyzed works date back more than ten years. Since then none categorical conclusion has been reached regarding the function of Transferrin as a modulator/ marker molecule of testicular function. In addition, studies related to iron transport have been performed in murine models. The murine model is very useful for studying many molecular and biochemical processes associated with the formation of sperm cells. These studies do not always correlate with those of humans. Due to the apparent evolutionary divergences in fertilization events, it is important to study the intervening structures in each particular species.22
In order to validate in humans the proposed specific synthesis of a Tf isoform by Sertoli cells in murine model we performed a Western Blot. Only measurements of seminal androgen-binding protein (ABP), another protein secreted by the testis, can be considered a functional marker specific to this structure. However, the ABP assay is time consuming and the concentration of this protein in semen is very low. For this reason, it would be very interesting if transferrin measurement could be considered as a possible indicator method of testis function.
Tf concentration was significantly lower in samples with low vitality and no correlation was found with morphology. Concentration of sperm cells was studied and compared with the samples´ transferrin concentration levels. (According to the WHO, the LRL is 15 million cells per mL). This study was highly significant since none of the selected samples, under LRL, reached Transferrin population mean. In addition, samples which did not reach LRL for progressive motile spermatozoa (32%) were selected, correlating them to transferrin concentration; we could observe that 85% did not reach the population mean.
We postulate that the decrease in the concentration of Tf in PS could be associated with abnormal spermatogenesis leading to a decrease in the number and mobility of spermatozoa. Other researchers23,24 have studied the antioxidant function of metal-transporter proteins which is believed to play an important role on the integrity and function of sperm cells. We are now studying the oxidative stress of sperm cells, evaluating peroxidation on the lipid membrane and/ or determining the concentration of superoxide dismutase in PS in order to obtain conclusive data.
In this study, only proteins involved in the metabolism of iron as biomarkers of testis function and its possible application for the diagnosis of male infertility are analyzed. Specific studies will be required to validate the biomarker status of other organs which also participate in the production of SP. Other types of markers deserve special attention given their potential predictive value for specific pathological situations, for example, in prostate cancers. Using an integrative genomics approach, we will identify candidate biomarkers for each of the organs involved in the reproductive process.
The studied biochemical markers were significantly different in seminal plasma and serum, and no relationship was found between them. Anomalies of many sperm cell functions might be due to the action of a number of factors. Knowledge of the pathophysiological mechanisms of these factors is of great importance for the treatment choice. A more in-depth study of TfT will allow, perhaps, considering it as a possible biomarker of testicular function
To the patients who participated in the project. To urologist doctor Esteban Streiger, for his collaboration in data collection. To Ms. Alejandra Olmos for her assistance in the statistical processing.
Authors declare that there is no conflict of interest.

©2021 Raspo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.