eISSN: 2379-6367


Research Article Volume 6 Issue 1
1Faculty of Pharmacy, Al-Quds University, Palestine
2Department of Life Sciences, Al-Quds University, Palestine
3Department of Earth and Environmental Sciences, Al-Quds University, Palestine
4IFBV-BELHERB, Luxembourg
Correspondence: Mutaz Akkawi, Department of Life Sciences, College of Science and Technology, Al-Quds University, Abu-Dies, Palestine , Tel 972(0)526435785
Received: November 16, 2017 | Published: January 4, 2018
Citation: Abu-Lafi S, Akkawi M, Abu-Remeleh Q, et al. Pure isolates and preparative hplc fractions or crude extract of Inula viscosa: effect on β-hematin inhibition in vitro. Pharm Pharmacol Int J. 2018;6(1):4-9. DOI: 10.15406/ppij.2018.06.00145
The antimalarial activity of wild Inula viscosa (I. viscosa) plant leaves was investigated. The efficacy of the whole crude extract versus preparative HPLC fractions versus pure isolates were compared by measuring their effect on β-hematin inhibition in-vitro. The preparative HPLC experiments consisted of reversed phase preparative column (22.2mm x 250mm, 10μm) and linear gradient of water, acetonitrile as the mobile phase. Injection volume was 1000μl and the wavelengths range was from 200-450nm using photodiode array detector (PDA). While fractions(IV, V and VI) showed antimalarial potential in comparison to chloroquine positive control, the rest of the fractions did not show any significant inhibition to the β-hematin formation. The antimalarial results showed that whole crude exact of the plant works better than the preparative fractions or the pure isolates presumably due to synergistic effect. The chemical identity of some of the pure isolates was explored using UHPLC-ESi-MS. Moreover, I. viscosa extract powder stayed stable ove r several years, while many other products such as Artemisia annua extract or Artemisin Combined Therapy (ACT) drugs rapidly lost their efficiency under tropical storage conditions.
Keywords: inula viscose, malaria, hemozoin, β-hematin, preparative HPLC, LC-MS
Malaria is still one of the largest medical threats to humans worldwide. Despite the global efforts to control it, many areas around the world remain malaria endemic, mainly confined to poor tropics and subtropics with huge global economic burdens.1 In 2015, the World Health Organization (WHO) estimated about half of the world population is at risk of this disease, malaria deaths are so frequent that statistically speaking every twominutes a child dies from it.2 Malaria parasites (Plasmodium) are introduced into human hosts by feeding female anopheles mosquitoes. There are five species that infect humans worldwide of these, Plasmodium falciparum is the deadliest.3 During the asexual part of the parasite life cycle (intra-erythrocytic stage), the Plasmodium feeds on the hemoglobin of the hosts RBC’s.4 This stage is responsible for the clinical symptoms of the disease and the end product of this consumption is the liberation of highly activated Ferriprotoporphyrin (IX) that can kill the parasites through cellular oxidative stress.5‒6 Unfortunately, the parasite has a solution to this problem by dimerizing this ferric heme into an insoluble more stable form called hemozoin or malaria pigment.7‒9 Purified hemozoin was found to be identical to the synthetic analogue, β-hematin, an insoluble form of ferric protoporphyrin IX (FP),10 produced in the presence of acetic acid11,12 which can be used as tool to test new potential antimalarials13,14 Due to indiscriminate use of drugs, an alarming increase in drug resistance emerges,15 which has become a global concern. Consequently, researchers are increasingly turning their attention to folk medicine and exploring natural resources for new bioactive compounds, looking for new leads to develop better drugs.16 According to the WHO, about 80% of the world’s population relies on traditional medicine.17
In recent years, resistance to artemisinin, the core compound in WHO recommended combination treatments for malaria18 has been detected in south East Asia,19 necessitating the urge for finding a new drug against this dreadful disease. Inula is a large genus containing about 120 species that belongs to the family Asteraceae. I. viscosa, also known as Dittrichia viscosa20 is a predominant Mediterranean herbaceous perennial plant that is traditionally used in Palestinian folk medicine with a large number of medical applications21,22 such as a diuretic, antifungal23 topical anti-inflammatory and hemostatic activity.24 Several other studies have shown antimicrobial activity,25 antioxidant,26 and anti-inflammatory,27 and hepatoprotective activities.28 I. viscosa contains a large number of pharmacologically active compounds. Previous studies isolated and identified various chemical compounds in Inula species including flavonoids29,30 terpenes,31 sesquiterpenoids32,33 and sesquiterpene Lactones;34 We have investigated the traditional Palestinian I. viscosa plant crude extract in the past and found it to exhibit potential anti-malarial activity in vitro.35 The current work however, focuses on the preparative HPLC fractionation of the alcoholic extract of I. viscosa and their in vitro effect on β-hematin inhibition as a parameter to antimalarial activity. Moreover, the full scan UHPLC-ESi-MS was conducted to reveal the structure of some isolated compounds probably responsible for the antimalarial activities.
Dimethyl sulfoxide (DMSO) of 99.5% purity, chloroquine diphosphate salt, sodium acetate of 99% purity and hemin chloride were procured from Sigma-Aldrich, Israel. Glacial acetic acid was purchased from Fluka. Ethanol (EtOH) solvents were purchased from Merck (Germany). Fresh wild leaves of Palestinian I. viscosa were collected towards the end of October 2015 from different areas around Jerusalem far from agricultural lands. Samples were air dried in the shade for 10days. Grinded and kept in a fridge.
Preparation of plant extract
The crude extract was obtained by macerating (1:10)wt/vol of dried plant leaves in 35% ethanol, left for 24 hours at room temperature, then filtered using MN 615.Ø110mm filter paper. The resultant extract was evaporated using (IKA WEREK RV06-ML) rotary evaporator at 70°C under reduced pressure. Then, it was lyophilized using Labconco freeze drier (USA), until constant weight was achieved. The final dried extract was stored in amber bottle and kept in the fridge until fractionated by preparative HPLC.
In vitro semi-quantitative test for screening of anti-malarial activity
As per the protocol described by Deharo et al.,36 a mixture containing 50μl of 0.5mg/ml haemin chloride (freshly dissolved in DMSO), 100μl of 0.5 M sodium acetate buffer (pH 4.4) and 50μl of the tested anti-malarial drug solution or control, was incubated in a normal non-sterile 96-well flat bottom plate at 37ºC for 18-24hours. It is imperative that the solutions are being added to the plate in this order. The plate was then centrifuged for 10minutes at 4000rpm. The supernatant was removed and the pH of reaction was measured. The final pH of the mixture should be between 5.0 and 5.2. The wells were washed with 200μl DMSO per well to remove free haemin chloride. The plate was centrifuged again, discharging the supernatant afterwards. The β-hematin remaining was then dissolved in 200μl of 0.1M NaOH to form alkaline hematin that could be measured spectrophotometrically. Finally, the absorbance was determined at 405nm using an ELISA reader (Stat Fax-2100). Ultrapure water was used as negative control, whereas chloroquine dissolved in ultrapure water was used as positive control.
Analytical HPLC, preparative HPLC and UHPLC-MS instrumentations
Analytical samples were analyzed using HPLC of Waters Alliance (e2695 separations module), which is equipped with 2998 Photo diode Array (PDA). Data acquisition and control were carried out using Empower 3 chromatography data software (Waters, Germany). The Preparative High Pressure Liquid Chromatography (Prep-HPLC) system consisted of 3535 quaternary gradient module equipped with 996 PDA detectors. The UHPLC was performed under reverse phase conditions using a TSQ Quantum Access MAX (Thermo Scientific, San Jose, CA, USA) which includes a Dionex Pump with a degasser module, an Accela PDA detector and an Accela Autosampler. The chromatographic separations were performed on a Kinetex™ column (C8, 2.6µm particle size, 100 Å pore size, 100 x 2.1mm) from Phenomenex, USA. A UHPLC Security Guard cartridge (C8, for 2.1mm ID column) was from Phenomenex, USA. The injection volume was 10μL; the oven temperature was maintained at 35°C.
Chromatographic conditions
Crude I. viscosa from 35% ethanol was run on reversed phase ODS column of Waters (XBridge, 4.6 ID x 150mm, 5μm) with a guard column of XBridge ODS (20mm x 4.6mm ID, 5μm). The mobile phase consisted of a binary solvent mixture of 0.5% acetic acid solution (A) and acetonitrile (B) in linear gradient mode. A 100% of solvent A was initially used and then descended to 70% A in 40minutes, then to 40% A in 20minutes, finally to 10% A in 2minutes and remained there for 6minutes and then back to the initial conditions in 2minutes. The HPLC system was equilibrated for 5minutes with the initial acidic water mobile phase (100% A) before injecting the next sample. All the samples were filtered with a 0.45μm PTFE filter. The PDA wavelengths range was from 210-500nm. The flow rate was 1ml/min. Injection volume was 10μl and the column temperature was at room temperature. The preparative HPLC experiments were run on ODS column (Agilent PrepHT C18, 22.2x250mm, 10μm). The gradient program started from 95% water, 5% acetonitrile to 100 % acetonitrile in 17minutes and back to the starting mobile phase composition in 4minutes. The flow rate was 12ml/minutes, the injection volume was 1000μl, the column temperature was set at room temperature and the PDA wavelengths ranged from 200-450nm. The UHPLC-ESi-MS chromatographic separation was achieved using a linear gradient program at a constant flow rate of 0.4 mL/min over a total run time of 70 min. The mobile phase gradient program was the same as the HPLC except for the use of 0.1% formic acid instead of acetic acid. Samples were detected by a TSQ Quantum Access Max mass spectrometer in positive and negative ion mode using Electron Spray ionization (ESi) and full scan acquisition. Air was produced (SF 2 FF compressor, Atlas Copco, Belgium). Purified nitrogen was used as source and exhaust gases.
I. viscosa preparative sample preparation
700 mg of crude Palestinian I. viscosa was dissolved in 15ml ethanol with 5 drops of acetic acid, and then the solution was directly filtered through PTFE 0.45μm membrane. One ml (46.6mg) of the clear solution was directly injected to the preparative HPLC column and nine fractions were collected. Fractions collected time were as follows: I (4-6.5), II (9.3-11.2), III (11.2-13.7), IV (13.7-19), V (20.5-23), VI (23-23.8), VII (23.8-24.5), VIII (24.5-25.5), IX (25.5-26)minutes.
It is known from previous literature that the chemical constituents from the genus Inula contains more than 117 different compounds including volatile, semi-volatile and non-volatiles polar compounds.28 This includes broad range of monoterpenes, sesquiterpenes, diterpenes, flavonoids, and glycolipid. Depending on the nature of the extracting solvent used, one can roughly predict the nature of the isolated compounds. In the current effort, 35% ethanol was utilized as a macerating agent at room temperate to extract out the polar non-volatiles phenolic compounds. The gentle extraction method used was to deliberately avoid the degradation of any thermo labile active compounds and to imitate as much as we can the traditional use of natural products simple extractions to cure malaria. We have previously investigated the crude raw material powder from 35% ethanol extract of I. viscosa and measured it’s in vitro inhibitory effect on β-hematin formation. The crude alcoholic extract of I. viscosa has the capability to impede the formation of β-hematin in-vitro; with an efficiency of about 93% when compared to the standard chloroquine which gave 94% at comparable concentrations.35 However, pure isolates or preparative HPLC fractions have never been explored yet. Surprisingly, I. viscosa crude ethanolic extract was found to be quite stable when left at room temperature for about four years by maintaining its in vitro efficiency (Figure 1). As in Figure 1, the absorption at 405nm of the same whole crude exact almost stayed the same over a period of more than three years. This is a very important finding because in comparison to the Artemisia annua extract, we noticed that the latter lost its antimalarial activity within fewdays .37 Stability problems are known to exist for some isolated pure molecules such as artesunate and amodiaquine as well38 especially under tropical climate hot and humid weather. This addresses a major concern in alternative therapy: in Southern countries the whole plant extract is usually used rather than isolated pure individual compounds and in the Western therapy isolated pure isolates are used exclusively. The stability of I. viscosa extracts may have another impact. The food industry is desperate for plant extracts which could have a stabilizing and anti-oxidant effect on their produces.
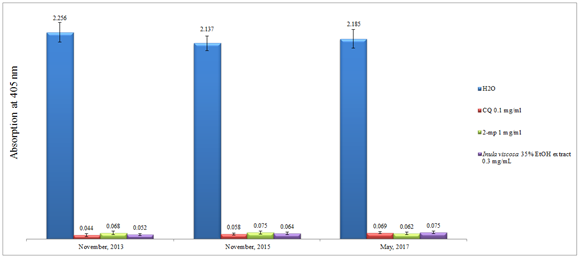
Figure 1 Column diagram representing the effect of time on the efficacy of potential anti-malarial drug 35% ethanol extract of I. viscosa leaves compared to the control water and positive controls chloroquine (CQ) 0.1 mg/mL, 2-mercaptopyrimidine (2-mp) 1mg/mL; showing the absorption values of dissolved β-hematin (alkaline hematin) at 405 nm using ELISA reader; according to E. Deharo semi-quantitative method; each result represents the average of 24 individual experiments.
The typical analytical HPLC-PDA chromatogram of the crude extract of I. viscosa (5mg/ml, 10μl injection) at 320 nm is shown in Figure 2. The preparative chromatograms of the 700mg crude extract (1000μl injection) at 320nm are shown in Figure 3. The analytical and the preparative chromatograms showed peaks that share the same maximum of UV-Vis wavelengths at 324-328nm and with a shoulder at 290-295nm. This observation indicates that the separated compounds share the same main functional groups, i.e., chromophores that are most likely belong to the flavonoids family. It is clearly seen that the major doublet peak in the preparative HPLC fraction (IV) that eluted at 16minutes is the same peaks eluted at 27.1-27.6minutes using the analytical mode. While preparative fraction III corresponds to the peak eluted at 14minutes. Three fractions (IV, V and VI) out of nine (I-IX) from the 35% ethanolic extracts of I. viscosa leaves were shown to possess in vitro effect on β-hematin inhibition. It is worthwhile mentioning that the fractions VI-VIII are representing isolated pure compounds (Figure 3). Absorption results obtained at 405nm were compared to positive controls of chloroquine (CQ) and amodiaquine (AD), at 0.1mg/ml each, and negative controls of ultrapure H2O and DMSO. According to the semi-quantitative method performed, the absorption is inversely proportional to fraction efficiency and to β-hematin content (Figure 4). Fraction IV that comprises two major compounds emerged together at retention interval of 13.7-19minutes (Figure 3) showed an absorption value of 0.221 at a concentration of 0.3mg/ml. The less active fraction V that eluted between 20.5-23minutes showed an absorption value of 0.648. Fraction VI which is a pure individual compound eluted at 23-23.8minutes (Figure 3) showed moderate activity (absorption of 0.338) in comparison to the positive controls (Figure 4). Although fractions VII and VIII are presumably pure isolates, nonetheless, they are inactive.
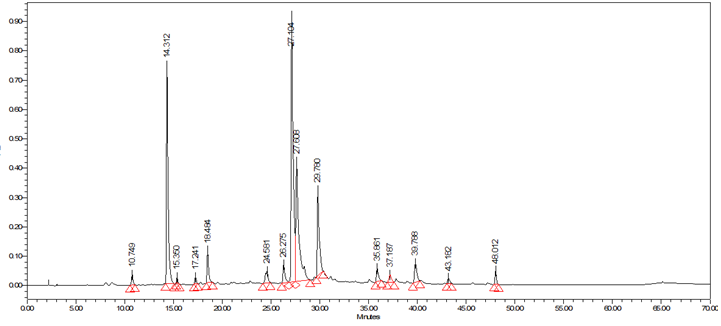
Figure 2 Typical HPLC-PDA chromatogram of all the peaks present in crude 35% ethanol extract of I. viscosa at 320 nm and their overlaid UV-Vis spectra between 210-500 nm.
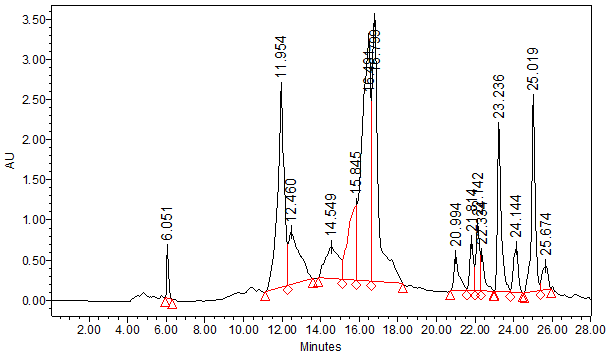
Figure 3 Typical preparative HPLC-PDA chromatogram of crude 35% ethanol extract of I. viscosa. 1 ml was injected at flow rate of 12ml/min at 320 nm using inch reversed phase column. Fractions collected time were as follows: I(4-6.5), II (9.3-11.2), III (11.2-13.7), IV (13.7-19), V (20.5-23), VI (23-23.8), VII (23.8-24.5), VIII (24.5-25.5), IX (25.5-26) minutes.
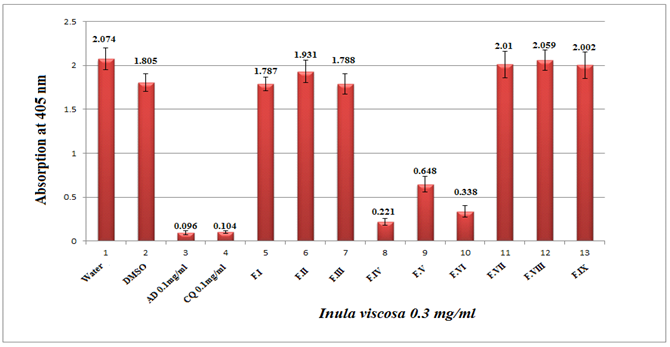
Figure 4 Column diagram representing the efficiency of nine fractions obtained from preparative HPLC separation of 35% ethanol extract I. viscosa leaf, dissolved in water-DMSO at concentration of 0.3mg/ml. The results are compared to negative and positive controls, showing the absorption values of dissolved β-hematin (alkaline hematin) at 405 nm using ELISA reader. Each result represents an average of 16 individual experiments.
In poor countries drinking herbal tea extract is affordable and cheap in comparison to pure drugs offered by western therapy. Although the whole crude extract contains individual antimalarial compounds or fractions at a much lower dose, the antimalarial activity is superior due to synergistic interaction between compounds present in the I. viscosa extract. Recent LC-PDA-ESI-MSMS investigation has characterized the presumed antioxidants and antifungal phenolic compounds in methanolic I. viscosa extract in the negative ESi mode.39 The phenolic profile generated was dominated by hydroxycinnamic acid, flavonols and flavanones derivatives. The most prominent components were mono- and dicaffeoylquinic acids.39 In order to explore the structural features of the phenolic compounds from the 35% ethanolic extract, a full scanned UHPLC-PDA-ESi-MS using the negative and positive electro spray ionization modes was examined. Figure 5 showed the UHPLC chromatogram of the major components at 320 nm. As seen in Figure 5, the major peaks at 10.97, 27.38, 28.42 and 31.05minutes are very similar to the major peak profiles of the analytical HPLC at 14.31, 27.16, 27.60 and 29.78 respectively (Figure 2). The major peak eluted at 10.97minutes (in fraction III) gave a deprotonated pseudo-molecular ion [M-H]- at m/z of 407.17 Da with a base fragment ion at 375.27 Da. Other major peaks appeared at retention of 16.5minutes showed molecular ion [M-H]- at m/z of 515.45 Da and a fragment at 353.28 Da presumably signifying to the caffeoylquinic acid (chlorogenic acid) skeleton which has a molecular weight of 354 Da (Figure 5). Other adjacent peaks at a retention time of 27.5 and 28.5minutes showed a deprotonated peak molecular ion [M-H]- at 515.45 Da and 405.31 Da and the same chlorogenic acid backbone base peak at 353.30 Da. The deprotonated 515.45 Da ion may indicate the presence of more than one caffeoyl group attached to different quinic acid OH groups. The same observation was seen in Mahmoudi et al.39 Both 27.5 and 28.5minutes peaks were eluted in fraction IV of the preparative HPLC fraction (Figure 3). The peak at 31minutes exhibited the same deprotonated molecular ion at m/z 515.45 and 353.33 base peak indicative of another geometrical isomer of dicaffeoylquinic acid. It is evident that all the major peaks showed a fragment at m/z of 353.30 Da which indicates that the isomeric flavonoids are a shared caffeoylquinic acid compound, which is the ester of caffeic acid and (-)-quinic acid, is known for its antioxidant activity.40 Fraction VI that eluted at 23-23.8minutes using preparative HPLC presumably is indicative of the presence of sakuranetin compound since it showed a protonated molecular ion [M+H]+ at m/z of 284.20 Da and a deprotonated molecular ion [M-H]- at m/z of 286.20 Da.
The emergence of resistant strains of Plasmodium falciparum to common antimalarial drugs, ranging from chloroquine to artemisinin combined therapy has been recognized. Development and search of novel and effective antimalarial agents to overcome chloroquine resistance have become an indispensable issue.
As unfold from the results of this study, three fractions (IV, V and VI) out of nine from the 35% ethanolic extracts of I. viscosa leaves were shown to possess in vitro effect on β-hematin inhibition in comparison to the positive controls. The crude whole extract versus the preparative HPLC fractions that includes pure compounds (VI, VII, VIII) were tested and the crude extract was found to be superior on β-hematin inhibition. This observation demonstrates that raw extracts sometimes exceed the efficacy of either the preparative fractions or isolated pure compounds due to synergistic effect.
None.
Author declares that there is no conflict of interest.

©2018 Abu-Lafi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.