eISSN: 2379-6367


Research Article Volume 5 Issue 6
1College of Science, University of Baghdad, Iraq
2College of Science for Women, University of Baghdad, Iraq
Correspondence: Mais E Ahmed, Lecturer, College of Science, University of Baghdad, Iraq
Received: October 30, 2017 | Published: December 7, 2017
Citation: Ahmed ME, Mossaw MTA. Production of purified mrsacin from local clinical strain of methicillin-resistant Staphylococcus aureus and its applicability as antimicrobial. Pharm Pharmacol Int J. 2017;5(6):219-224. DOI: 10.15406/ppij.2017.05.00142
One hundred fifty clinical samples (burns, wounds, urine, nasal and ear swabs) were collected from patients of Al-Yarmouk Hospital and Teaching Baghdad Hospital during the period from November/2015 to January/2016. Therefore was aimed to produce of Crude and purified bacteriocins from antibiotic resistant bacteria (G-ve)MRSA isolated from clinical samples and determine the optimization conditions for production of bacteriocin and their purification and characterization. Cultural and morphological characteristic examination, biochemical tests were conducted and confirmed the diagnosis by API Staph and antibiotics sensitivity test confirmed by Vitek-2 system. The results showed 102(68%) isolates of S. aureus, all of which were Methicillin Resistance S. aureus (MRSA), constituted 50% from the ratio in the burns and wounds samples, while the lowest 13(12.7%) isolates were from ear samples, Vitek 2 system gave confirmation of positive results for MRSA with a probability 98-99%. The MRSA isolate S12 was chosen among five bacterial isolates as a good producer for crude MRSAcin according to their widest inhibition zone by Five methods were used to detect of MRSAcin production by (well diffusion assay WDA, flip-streak, spot on the lawn, Cup disc and paper disc method), against indicator pathogens (G+ve, G-ve bacteria and yeast) The optimum conditions for MRSAcin production were in trypticase soya broth with pH 7, at temperature 37°C for 48hrs and inoculums size of bacterial culture 6×108cell/ml. "Bacteriocin after Purification was made by (two steps) method extraction by ammonium sulphate at 70% next step by DEAE-cellulose ion-exchange chromatography and Sephadex G-50 gel filtration chromatography. Also studied of Physical characteristics and the molecular weight showed of MRSAcin (15) KDa by (SDS-PAGE) Sodium Dodecyl Sulfate-polyacrylamide gel electrophoresis.
Key words: MRSA, bacterocin, vitek 2, pathogenic bacteria
Strains MRSA that acquired a specific gene makes for resistant to most (beta-lactam) antibiotics. Also common resistance to other antibiotics especially in hospital-associated, these organisms microbiology are nosocomial pathogens serious and finding an effective treatment can be challenging. Community associated MRSA strains are outside hospitals originated having moved into hospitals and become resistant to drugs increasing than beta-lactams.1 (Methicillin-resistant Staphylococcus aureus) resistant usually to several type antibiotics and also resistance to ß- lactam and a particular shows ability to spread in most hospitals and present now in most countries of Iraq.2 Risk factors for the hospital acquisition of MRSA include prolonged hospitalization that stay in an intensive care unit, chronic diseases such as chronic renal failure and malignancy, prior exposure to antibiotics, surgery and contact with a patient known to be colonized or infected with MRSA.3 Bacteriocins are antimicrobial peptides or proteins ribosomally safe substances that are non-toxic to eukaryotic cells. Bacteriocins bacterially produced natural peptides released by different varieties of bacteria and archea that are active against other bacteria and the producer has a specific immunity mechanism.4 Bacteriocins which have inhibitory effects towards sensitive strains are produced by both gram-positive and gram-negative bacteria, Bacteriocins from lactic acid bacteria are used in food perecierpitive like in dairy products. It has a useful use in health care products and cosmetics for treatment of acne, also being used inhibition of dental caries in toothpaste and mouthwash for the periodontal diseases.5 Staphylococcin Bac188 also showed very potent activity against many clinical isolates of Mycobacterium tuberculosis. Staphylococcin Bac188) Staphylococci are known to produce a wide variety of inhibitory substances.6 Other antimicrobial substances produced by staphylococci have been categorized as true bacteriocins.7
Isolates bacterial and culture media
Confirmation of S. Aureus: The samples collected on mannitol salt broth media were incubated at 37°C for 24h and then inoculated onto mannitol salt agar media and incubated at 37°C for 24h. Selected isolates with colony morphology, Gram stain reactions and biochemical characteristics (i.e., Coagulase, Catalase reagent, Oxidase reagent).
Confirmation of MRSA and antibiotic resistance testing: All isolates of S. aureus that demonstrated any level of Oxacillin (Methicillin) and Cefoxitin (FOX) resistance. All cultures were grown on Mueller-Hinton agar plates at 37°C for 18hours in the presence of the following antibiotics: Penicillin (10μg), Cefotaxiitin (30), Cefoxitin (30μg), Chloramphenicol C (30), Cefotaxime, CTX (30), Methicillin, ME(5) Oxacillin ,OX(1), Erythromycin, E (15) and Gentamycin ,CN (10) Tetracycline, TE(30) Vancomycin VA (20) (Diagnostic Systems). The inhibition of zone by (mm) each around disk compared and documented with a standard interpretive chart. The zone of inhibition (in mm) around each disk was documented and compared with a standard Clinical and Laboratory Standards Institute.8
Vitek 2 System: A specific number of the bacterial isolates were selected to confirm their identification susceptibility using the Vitek 2 system.
MRSAcin activity assay
The MRSAcin producer was The MRSA S12 isolate from infections wounds against indicator organism was S. aureus, E.coli and C. albicans obtain from (Department of Biology, College of Science, Baghdad, Iraq) the medium culture used for MRSAcin production in liquid medium (TSB).9 In vitro antibacterial activity of Crude MRSAcin were examined for inhibitory activity against different strains of bacteria using (AWD) assay. MRSAcin of Measurement by serial two-fold dilutions of (crude MRSAcin) cell-free supernatant. Detect the antibacterial by dilutions were used to activity of bacteriocins against indicator bacteria by agar well diffusion assay.10 AU: was defined the arbitrary unit as the reciprocal of the highest dilution shows a zone clear of inhibition growth of the isolate test. AU was calculated as: (1000/100) × D, where 1000: constant, 100: volume of supernatant in a well (µl) and D: the dilution factor.11
MRSAcin concentration
It was determined follow12 the concentration assay and activity of (MRSAcin) bacteriocins determined.
Bacterocin was inoculated with 2% of (6×108 cell/ml) and at 37°C incubated over night13 were harvested the Cells by centrifugation at (6000 rpm for 15min) the supernatant as crude (MRSAcin) extract (CME) and heated at 80°C for 10min then cooled at last centrifuged at 6000rpm.14 The crud was mixed thoroughly with (Ammonium sulphat) a ratio 70%.The mixture at 4000rpm for10minutes was centrifuged separation then the 20mM sodium citrate buffer (pH 7) sediment was re-suspended.15 Chromatography gel (DEAE-Cellulose) was used as a first step for purification (MRSAcin). The MRSAcin was eluted from the column (2×40) cm diameter by 20mM sodium citrate buffer (pH 7). Activity and absorbance at 280nm of each fraction were determined, the active fractions were mixed & next step by (gel filtration chromatography) sephadex G-50.
Determination of the molecular weight
The purified MRSAcin and its molecular weight were estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).16
Effect of pH MRSAcin
Was mixed solution with 10mM (potassium phosphate buffer)at pH values ranging from (2 to 12, After 30minutes tested for remaining activity by incubation at 37°C the samples were re-adjusted to pH 7 with 1N NaOH or 1N HCl.
Effect of temperature
It was assayed by treating (MRSAcin) solution at (20, 25, 30, 37 and 40) °C and activity was assayed after (10, 30 and 60) min at each of these temperatures. Activity also assayed after 15minutes at 121°C.
Estimation of protein by lowry’s method
The samples were analyzed for protein using Lowry’s method. 5 tubes which serve as standard and one tube for the supernatant and one tube for the pellet were taken and 0.2ml, 0.4ml, 0.6ml, 0.8ml and 1ml of protein solution were added to the standard tubes marked as 0.2ml of supernatant and 0.8ml of pellet were also added to the respective tubes and each of the tubes were made up to 4ml by adding water. Then 5.5ml of reagent C to all the tubes was added and at room temperature kept for 10-15 mins. Then 0.5ml of reagent D was added to all the tubes and kept in dark for 30mins.
Antibiogram Profile MRSA Isolated Clinical Samples the results showed 102(68%) isolates of S. aureus, all of which Methicillin Resistance S. aureus (MRSA) were, constituted 50% from the ratio in the burns and wounds samples, while the lowest 13(12.7%) isolates were from ear samples. Highly significant differences (P˂0.01) recorded in both isolates among clinical samples. The isolates showed multi-resistant to Methicillin, Penicillin G, Cefoxitin, Erythromycin, Oxacillin, Chloramphenicol and Tetracyclin, while sensitive to vancomycin (Figure 1). These results agree with17 who found the resistance to vancomycin was (24.41%) in America. Clindamycin in the present study showed resistance rate 66 (64.70%). This result was similar to that obtained by Al-Dahbi AM.18
Vitek 2 system
Gave confirmation of positive results for MRSA and as a selected organism with a probability 98-99%19 showed that out of S. aureus isolates had oxacilin-sensitive/cefoxitin-resistant profile with a sensitivity of 88.7% and a specificity of 99.5% for the identification of MRSA isolates.
3-2 screening for crude MRSAcin
The MRSA isolate S12 was chosen among five bacterial isolates as a good crude MRSAcin producer according to their widest inhibition zone that reached 15mm on the basic indicator isolates (Figure 2).

Figure 2 Screening of crude MRSAcin from MRSA against E.coli O157:H7on sorbitol agar at 37°C for 24-48 hrs.
Comparison between antimicrobial activity for crude bacteriocin using agar methods
Comparing the five methods used in this research, all the methods showed antagonisms between bacteria, Table 1 shows antibacterial activity MRSAcin against indicator bacteria of G-ve, G+ve and yeast. was seen WDA as the best method to give higher diameter of inhibition zone (17mm) then spot-on the lawn method (16mm) and these two methods recorded significant differences (p˂0.05) compared with other methods. The present results agree with20 who found the well diffusion assay as the best method to identify the antagonism of microorganisms and spot-on lawn method was the worse (Figures 3‒5).
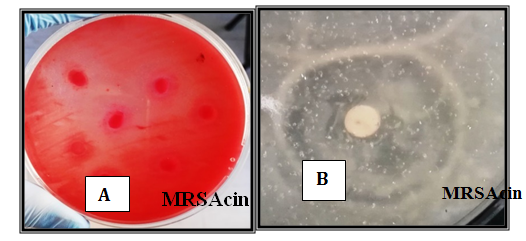
Figure 3 Antimicrobial activity of crud MRSAcin, MAR-pyocin and synergistic against Muller hinton agar for 24-48 hrs at 37°C

Figure 4 Antagonistic activity on Muller Hinton Agar for 24-48 hrs at 37°C againstMRSAandE .coliby A: Paper disc method; B: Flip-Streak Method (1) Crud MRSAcin.

Figure 5 Antimicrobial activity against A-Staphylococcus aureus B-C.neofurmous, 1: crud MRSAcin; 2: Purified MRSAcin on Mannitol salt agar and Sabouraud agar for 24-48 hrs at a 37°C by WDA.
Pathogenic Microbe |
Bacteriocin |
Well Diffusion |
Spot on the Lawn |
Cup Disc |
Paper Disc |
Flip Streak |
LSD Value |
Escherichia coli |
MRSAcin |
16 ± 0.07a |
14 ± 0.06ab |
10 ± 0.06bc |
7 ± 0.02cd |
6 ± 0.03d |
3.64* |
Staphylococcus aureus |
MRSAcin |
16 ± 0.05a |
15 ± 0.06ab |
12 ± 0.05bc |
9 ± 0.04cd |
7 ± 0.04d |
3.64* |
Candida Albicans |
MRSAcin |
16 ± 0.07a |
14 ± 0.06a |
8 ± 0.03b |
6 ± 0.02b |
5 ± 0.04b |
3.26* |
Table 1 Antimicrobial activity for Crude bacteriocin against Gve+ Gve– bacteria and Candida albicans.
LSD* each value is the mean of three replicate (mean ± SE) values with difference letter have significant differences.*(P<0.05)
Production of MRSAcin
CME was too denaturant any proteases heated. (Ammonium sulphat) extraction complete recovery of bacteriocins activity, extraction by ammonium sulphate 70% shown inhibition zone diameter reached 15mm (Figure 6). The result reported by Ge J et al.21 found that partial purification with ammonium sulfate precipitation bacteriocins have antimicrobial activities against food-spoiling bacteria and food-borne pathogens. These results indicated that the MRSAcin by MRSA were under the present condition carries a negative charge opposite to ion exchange charge and binding with exchanger resin. Fractions which have activity collected and concentrated. The specific activity of MRSAcin reached 1000 AU/mg with 2.8 purification fold and bacteriocin yield (34)% respectively (Figure 7). Ion exchange (DEAE-Cellulose) features used in this step due to high resolution power, easily prepared, high capacity, possibility of reactivate and uses many times as well as the simplicity of the principle of separation, which mainly depends on net charges of protein.22
Peaks of bacteriocins appeared maximum activity of MRSAcin when assayed against G-ve, G+ve pathogenic bacteria. The fractions 12 were observed was (a specific activity) for these fractions was 1200AU/mg protein with (1.2) purification folds (Table 2). The result showed that there is significantly (P≤0.05) decrease in the volume of purified MRSAcin when treat sephadex G-50 and DEAE-cellulose when compared with (NH4)2SO4 and culture filtration, in contrast, there was a significance increases in the activity of fold purification (Figure 8).23
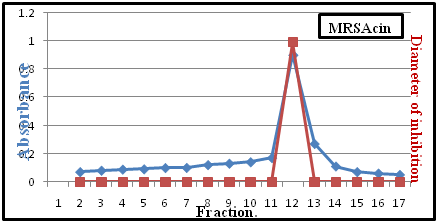
Figure 8 Purification of MRSAcin by gel filtration chromatography, (column Sephadex G-50) with dimensions (2x60) cm.
Purification Step |
Volume (ml) |
Activity (AU/ml) |
Protein Concentration (mg/ml) |
Specific Activity (AU/mg) |
Total Activity (AU) |
Fold Purification (Per Step) |
Yield (%) |
Culture filtrate |
200 a |
200 b |
0.9 a |
222 c |
44400 a |
1 b |
100 a |
(NH4)2SO4 precipitate |
150 b |
210 b |
0.8 ab |
263 c |
39300 b |
1.2 b |
89 b |
DEAE-cellulose |
15 c |
600 a |
0.6 bc |
1000 b |
15000 d |
3.8 a |
34 c |
Sephadex G-50 |
15 c |
600 a |
0.5 c |
1200 a |
18000 c |
1.2 b |
18 d |
LSD value |
18.21* |
91.52* |
0.262* |
84.74* |
761.3* |
2.509* |
9.31* |
Table 2 The purification steps of MRSAcin from MRSA.
Mean have difference latter, significant different. *(P<0.05).
Determination of molecular weight
The slandered protein was used as compared to detection molecular weight of MRSAcin, 0. As shown in Figure 9 the high molecular weight was found to be 15KDa, particularly for MRSAcin. The current results agree with molecular weight of both pyocin and bacillin extracellular bacteriocin were approximately 40 KDa according to reference protein ladder band, while the molecular weight of LB were estimated to be in the range of (10 to 25) KDa.24
pH stability of MRSAcin and MAR-pyocin
Figure 10 shows that Bacterocin were stable at pH values (4-8) remained active, while at pH values 9, the MRSAcin lost 50% of its activity.The whole activity was lost at the pH values 10, indicating its sensitivity to alkali treatment. The result showed by Naz S et al.,25 of pH on the biological activity, pyocin SA189 remained stable within the pH range (2 to 10).

Figure 9 SDS-PAGE Tricine-SDS-PAGE of bacteriocins, A: Molecular weight marker; B: Purified MRSAcin.

Figure 11 Residual activity of purified, A: MRSAcin at different temperatures for 60 min except at 121°C for 15 min.
Thermo stability for MRSAcin
Thermo stability for purified MRSAcin was assayed at different temperatures. As shown in (Figure 11), the MRSAcin was resistant to treatments with (50 and 75)°C for (10 and 30)min. At 100°C for (10 and 30)min, also appeared not thermo stability. However, 50% activity was lost after 30min at 100°C and after autoclaving; the result was agreement with.26
None.
Author declares that there is no conflict of interest.

©2017 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.