eISSN: 2379-6367


Research Article Volume 10 Issue 6
1Department of Pharmacology and Clinical Pharmacy, University of Tripoli, Libya
2Department of Medicinal Chemistry, University of Tripoli, Libya
3Department of Pharmacognosy, Faculty of Pharmacy, University of Tripoli, Libya
4Department of Chemistry, Faculty of Science, University of Tripoli, Libya
Correspondence: Fathi Mohamed Sherif, Ph.D., Professor of Pharmacology, University of Tripoli, Libya, Tel + 218 91 211 7258
Received: November 16, 2022 | Published: November 30, 2022
Citation: Bazine HA, Shlaka MA, Mezogi JS, et al. Phytochemical and pharmacological studies of Lycium schweinfurthii methanolic leaves extract (Solanaceae) in mice. Pharm Pharmacol Int J. 2022;10(6):201-206. DOI: 10.15406/ppij.2022.10.00386
Lycium schweinfurthii is found in stony and sandy places belonging to the Solanaceae family which is widely distributed in North Africa and Mediterranean area. The leaves and fruits of this plant have traditionally been used for gastrointestinal diseases. This study was aimed to evaluate the neuropharmacological activity of the methanolic extract of Lycium schweinfurthii in experimental animals. The methanolic extract of the leaves was prepared by the fractionation technique. Male Albino mice of 22.2 ± 2.1 gm were used to evaluate the activity of the extract by Irwin primary screening test, diazepam and thiopental -induced sleep and open field apparatus for general locomotor activity as well as analgesic activity was evaluated by acetic acid-induced writhing and hot-plate. The phytochemical screening was performed by qualitative and quantitative analysis. Diazepam at 2 and 20 mg/kg, flumazenil at 2 mg/kg, thiopental at 40 mg/kg, diclofenac at 25 and 50 mg/kg and indomethacin at 20 mg/kg were all used as references. The selected dose of 400 mg/kg of the methanolic extract was selected after the Irwin screening test. Lycium schweinfurthii extract significantly prolonged the duration of diazepam-induced sleep without significant difference in the onset of sleep. Pretreatment with flumazenil completely blocked the hypnotic effects of the plant extract and diazepam. The plant extract with thiopental did not produce any significant change in the onset and duration time of sleep. In an open-field test, a combination of the plant extract with diazepam produced a significant reduction in both exploratory and general locomotor activity which was observed in four min and 30 min, respectively. The plant extract significantly induced a profound central and peripheral analgesic action. While, the phytochemical screening showed presences of flavonoids, tannins, saponins, phenols, cardiac glycoside, alkaloids and carbohydrates in the extract. The total flavonoids were the most content compounds in the extract. Thus, these findings indicate that Lycium schweinfurthii has depression, sedative and analgesic like activities.
Keywords: analgesic, behavior activity, diazepam, medicinal plant, mice, Solanaceae
Lycium schweinfurthii dammer is a shrub belonging to the Solanaceae family which is commonly spread in North Africa and Mediterranean regions. This plant grows in stony and sandy areas along the coastal line. It is found in some countries as Tunisia, Libya, Portugal, Egypt and Spain.1 Traditionally, the leaves and fruits of Lycium schweinfurthii were used to treat some gastrointestinal diseases such as peptic ulcers.2 A previous phytochemical screening reported a presence of important bioactive compounds in different parts of L. schweinfurthii such as alkaloids, saponins, flavonoids and tannins. The total flavonoids and other contents were found to be more concentrated in the leaves than in other parts of the plant, and five flavonoids were isolated from the methanolic extract of the leaves using various chromatographic techniques.3 A novel glycoside and five previously identified compounds that were separated from L. schweinfurthii of the total plant methanolic extract showed a significant inhibitory activity and a reducing effect in postprandial hyperglycemia in diabetic patients.4 L. schweinfurthii was isolated with twenty-six known and two novel compounds, four showed cytotoxic effects against skin cancer cells and three showed cytotoxic effects against colon cancer cells.5 Our ongoing studies showed that the plant extract has a potent pharmacological activity in experiment animals, including anti-convulsion action (unpublished data). Diazepam belongs to benzodiazepine group which has widely been used to treat general anxiety disorders, muscle relaxant and used as sedative, hypnotic and anticonvulsant.6 Diazepam acts as facilitating the activity of gamma-aminobutyric acid (GABA) at various sites. In the central nervous system, it binds at an allosteric site at the GABA receptor leads to an increase in chloride ions influx resulting in hyperpolarization of the neuronal membrane, subsequently enhancing depression.6 Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used for their anti-inflammatory and analgesic effects. NSAIDs function through the inhibition of the cyclo-oxygenase enzyme (COX) which blocks the cascade of arachidonic acid to prostaglandins.7 To the best of our knowledge, there is no published data yet about the biological and pharmacological effects of this plant. Thus, the aim of this study was to explore the neuropharmacological activities of L. schweinfurthii leaves in mice.
Plant collection
The leaves of Lycium schweinfurthii (L. S) were collected from the city of Gharyan area, Northwest of Libya, in March 2021. The plant was identified by Dr. Mohammed Makhlouf. (Faculty of Science, University of Tripoli, Tripoli, Libya) and a voucher specimen of 676271 was deposited in the Herbarium of Department of Botany.
Plant preparation and extraction
The shade-dried leaves plants of L. schweinfurthii and ground into powder (350 gm) were extracted by cold maceration beginning with the solvent of least polarity to increasing (petroleum ether 40-60°C, ethyl acetate and methanol 96.0%) were partitioned as a first purification step for five days. Each solvent with intermittent shaking and stirring and finally, the methanol extract was obtained. The methanol extract was filtered and dried in a rotary vacuum evaporator at 40°C under reduced pressure to obtain the crude extract of 40.3 gm (yield 11.5%) and stored in a refrigerator at 4°C until used.8
Experimental animals
Male Albino mice weighing 22.2 ± 2.1 gm of four months old were used throughout this study. Mice were obtained from a local animal house (University of Tripoli, Tripoli, Libya). The mice were housed in standard cages (n = 6, for each cage) under normal laboratory conditions (temperature: 23 ± 2°C, humidity: 65-75% and light with 12 h dark/light cycle, 6: 00 am - 6: 00 pm). Animals were allowed a free access to water and standard food. Mice were acclimatized to laboratory conditions for two weeks prior to experimentation. All the animals were fasted overnight before the experiment but still allowed a free access to water. The experimental protocols were approved by University Ethics Committee, University of Tripoli (19152/2022) according to International guidelines of animal use. The plant extract was freshly prepared daily before the experiments and immediately used. All the treatments were administered by an intraperitoneal route of administration.
Phytochemical screening
The preliminary phytochemical screening of the methanolic leaves extract of L. schweinfurthii to detect qualitatively presence of any active constituent is used according to previous methods.9 This established procedures are used to screen of flavonoids, phenolic, alkaloids, anthraquinone, tannins, saponins, resins, cardiac glycosides, steroids and carbohydrates.
Total phenolic content
The plant phenolic content was measured by using Folin-Ciocalteu colorimetric method.10 A total of 200μl of the plant extract (0.1 g/ml) was used in triplicate. Then one ml of Folin Ciocalteu reagent (1:2 with water) was incubated for five min at room temperature (24 ± 2°C). One ml of 07.0% Na2CO3 was added to the solution and left to stand again for 90 min at room temperature. Afterwards, the absorbance of the extract solution was measured at a wavelength of 750 nm using a UV-VIS Spectrophotometer. The total phenolic content is expressed as milligrams of gallic acid equivalent per gram of extract.
Total flavonoid content
The plant flavonoid content was measured according to previous method.11 One ml of plant extract (0.1 g/ml) was diluted with four ml of water and then added 0.3 ml of NaNO2 (05.0% w/v). After five min, added 0.3 ml of AlCl3 (10.0% w/v) was followed by the addition of two ml of NaOH (1.0 M) six min later. The total was made up to 10 ml by adding 2.4 ml of distilled water and the sample was incubated at room temperature (24 ± 2°C) for 15 min. The absorbance was measured at 510 nm using a UV-VIS Spectrophotometer. The total flavonoid content extract is expressed as milligrams of rutin equivalent per gram of extract.
Total condensed tannin
The content of tannin in the plant was measured according to the previous method.12 A 50μl from the plant extract was mixed with 1.5 ml of 04.0% vanillin (prepared with methanol) and then added 750 μl of concentrated HCL. The solution was stirred vigorously and left in a dark at room temperature (24 ± 2°C) for 20 min. The absorbance was measured at 500 nm using a UV-VIS Spectrophotometer. The standard curve was prepared with tannic acid and the findings were expressed as milligrams of tannic acid equivalents per gram of extract.
Irwin primary screening test
This method was carried out according to the primary observation procedure originally described by Irwin in 1968.13 Male mice have been kept withinside the experimental environment to acclimate and randomly divided into five groups (n = 4, each group). Group 1 was given a vehicle and used as a control group. The other four groups were administered with the plant extract at doses of 200, 400, 800 and 1600 mg/kg, respectively. The mice were placed individually in a transparent cage to assess the influence of plant extract on behavioural, neurological and autonomic as explained in the Irwin test.13 Observations were made just by scoring of 0 to 8 instantly after intraperitoneal administration (0, 15, 30, 60, 120, 180 min, 24 hrs and 48 hrs). Mice were compared with the control group for any abnormal change in the behaviour and for the mortality.
Diazepam-induced sleep
Two groups of mice (n = 6, each group) were used in this experiment. Group 1 was given a vehicle and is used as a control group. Group 2 was given the plant extract at a dose of 400 mg/kg. After 30 min, 20 mg/kg of diazepam was injected into both groups. The onset of sleep (the time taken to loss a righting reflex) and duration (interval between the loss and recovery of righting reflex) were recorded for each group.14
Flumazenil for diazepam-induced sleep
Two groups of mice were used (n = 6, each group). Group 1 was pre-treated with flumazenil (2 mg/kg) and after 30 min, given diazepam (20 mg/kg). Group 2 was administered flumazenil (2 mg/kg) and after for 30 min, the plant extract (400 mg/kg) and diazepam (20 mg/kg) were administered.15
Thiopental-induced sleep
Mice were divided into two groups (n = 6, for each group). Group 1 was given vehicle and is used as a control group. Group 2 was treated with plant extract (400 mg/kg). After 30 min, 40 mg/kg of thiopental was injected to two groups. Then animals were observed for the onset of sleep (the time taken to loss a righting reflex) and duration (interval between the loss and recovery of righting reflex) induced by thiopental.16
Open field test
The apparatus consists of a fiberglass closed arena (60 cm x 60 cm) with a 20 cm sidewall height. The arena was subdivided into 16 equal squares (15 x 15 cm). The open field test is used as a model to record the general locomotor activity and the exploratory behaviour of the animal. The parameters measured are horizontal movements (number of squares crossing in the central and peripheral lines) and vertical movements (rears in the central and peripheral by standing on hind limbs). Four groups of mice (n = 6 for each group). Group 1 was given vehicle and is used as a control group. Group 2 was administered the plant extract (400 mg/kg). Group 3 was given diazepam (2 mg/kg) as a standard group. Group 4 was given the plant extract (400 mg/kg) and diazepam (2 mg/kg). After 30 min of administration, each mouse was placed individually in the centre of the apparatus and observed for four min, with light and sound control. A similar test was repeated and observed for 30 min. All the above mentioned parameters were recorded by video camera as previously reported.17,18
Hot plate method of analgesia
This method is used for evaluation of the central analgesic activity of the extract by Eddy’s method.19 Six groups of mice (n = 6, each group) were used in this experiment. Group 1 was given a vehicle and used as a control group. Group 2 was given only plant extract at a dose of 400 mg/kg. Group 3 was treated with 50 mg/kg of the diclofenac sodium as a standard group.20 Group 4 was given a combination of plant extract (400 mg/kg) and diclofenac sodium (50 mg/kg). Group 5 administered 20 mg/kg of indomethacin used as the standard group.21 Group 6 was given a combination of plant extract (400 mg/kg) and indomethacin (20 mg/kg). The reaction time (paw licking or jumping) was recorded for each mice after 30 min from the administration of the plant extract and standard groups. A cutoff of 30 seconds was used in this test to avoid harmful effects for the mouse.
Writhing test
This method described by Koster and others,22 it is used for the 0.70% acetic acid-induced writhing test. The observation of contraction of abdominal muscles followed by the extension of the hind limbs is considered as writhing response. Mice were divided into four groups (n = 6 of each). Group 1 was given a vehicle and is used as a control group. Group 2 was given the plant extract at a dose of 400 mg/kg. Group 3 was administered diclofenac sodium (25 mg/kg) and is used as a standard group. Group 4 was treated combination of plant extract (400 mg/kg) and diclofenac sodium (25 mg/kg). After 30 min, acetic acid was given for each group. The number of writhing response was counted after five min of acetic acid injection for a period of 20 min.23
Statistical analysis
Data were expressed as mean±SEM. All the data were tested for parametric or non-parametric distribution by Kolmogorov-Smirnov test. An overall differences were analyzed by analysis of variance (one way-ANOVA) followed by Tukey’s post hoc multiple comparison test. Independent-samples t-Test was used. Non-parametric data were analyzed statistically by means of Mann-Whitney-U test. A p-value < 0.05 was considered statistically significant.
In this study, a dose of 400 mg/kg of the plant extract was chosen according to the pilot experiments of dose-response curve (data not shown). In table 1, the findings of the tests performed in preliminary phytochemical screening of methanolic leaves extract of Lycium schweinfurthii revealed presence of flavonoids, tannins, saponins, phenols, cardiac glycoside, alkaloids and carbohydrates.
Test |
Observation |
Finding |
Flavonoids (Shinoda) |
Pink colour |
+ |
Phenolic compounds |
Dark green colour |
+ |
Alkaloids: |
||
A- Dragendorff’s reagent |
Reddish-brown precipitate |
+ |
B- Mayer’s reagent |
Creamy-white precipitate |
+ |
Anthraquinone (Borntrager’s) |
- |
|
Tannins |
Ggreen colour |
+ |
Saponins |
Permanent foam |
+ |
Resins |
- |
|
Cardiac glycosides (Keller-kiliani) |
Reddish-brown layer |
+ |
Steroids (Salkowaski) |
- |
|
Carbohydrates: |
||
A- Fehling’s test (reducing sugars) |
Brick-red precipitate |
+ |
B- Molish’s Test |
Violet ring at the interphase |
+ |
Table 1 Preliminary phytochemical screening of L. schweinfurthii leaves extract
+, indicates presence of the constitute; −, indicates absence of the constitute
Figure 1 shows the quantitative phytochemical analyses of the plant extract. It is revealed the presence of high concentration of total flavonoids in the extract. Both phenolic and tannin are present in low concentrations.
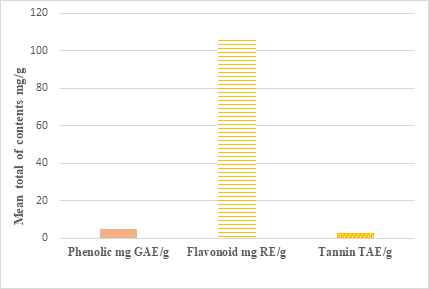
Figure 1 Phenolic, flavonoid and tannin contents of L. schweinfurthii leaves extract.
Data are mean ± SD.
In table 2, the plant extract at doses of 200, 400 and 800 mg/kg does not have a significant impact on the awareness but at doses of 400 and 1600 mg/kg, a marked increase in the passivity and a decrease in visual placing was observed. Further, a decrease in the spontaneous activity and reactivity were noted. However, at doses of 200 and 800 mg/kg, an increase in the stereotypy (head flicking and pacing bipedal) and a marked increase in the spontaneous activity and reactivity at a dose of 200 mg/kg were observed. The plant extract showed at a dose of 1600 mg/kg staggering gait with an abnormal gait as well as an appearance of the slight tail elevation. The plant extract at a dose of 400 mg/kg narrowed the palpebral opening. Administration of the plant extract did not have any lethal effects over a period of 24 hrs observation.
Profile |
Basal score |
200 mg/kg |
400 mg/kg |
800 mg/kg |
1600 mg/kg |
Control |
Alertness |
4 |
4 |
4 |
4 |
3 |
4 |
Visual placing |
4 |
4 |
2 |
4 |
1 |
4 |
Passivity |
0 |
0 |
1 |
0 |
3 |
0 |
Stereotypy |
0 |
2 |
0 |
1 |
0 |
0 |
Grooming |
4 |
4 |
4 |
4 |
4 |
4 |
Spontaneous activity |
4 |
6 |
2 |
4 |
1 |
4 |
Reactivity |
4 |
6 |
2 |
4 |
1 |
4 |
Staggering gait |
0 |
0 |
0 |
0 |
2 |
0 |
Abnormal gait |
0 |
0 |
0 |
0 |
2 |
0 |
Straub tail |
0 |
0 |
0 |
0 |
2 |
0 |
Body posture |
4 |
4 |
4 |
4 |
1 |
4 |
Palpebral opening |
4 |
4 |
3 |
4 |
6 |
4 |
Table 2 Behavioral, neurological and autonomic profile of Lycium schweinfurthii leaves extract in mice
Peak effect of the extract on each of the dose listed is determined on scale ranging from 0 to 8.
As shown in figure 2, the methanolic extract of the plant (400 mg/kg) with diazepam produced a significantly increase by 193% in duration of sleep but with no significant difference in onset of sleep compared to the control group. Administration of plant extract at a dose of 400 mg/kg in combination with thiopental 40 mg/kg did not produce a significant change in onset and duration time of sleep compared to the control group. In both groups, administration of flumazenil in a dose of 2 mg/kg did not produce loss the righting reflex.
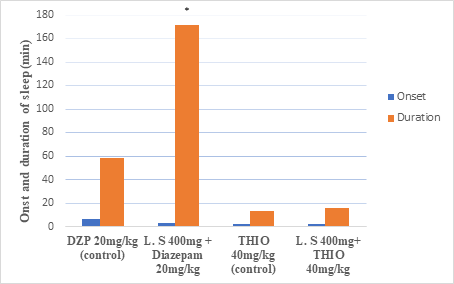
Figure 2 Effect of Lycium schweinfurthii extract on diazepam and thiopental-induced sleep in mice.
Data are Mean ± SEM. *Significant difference from the control, DZP is diazepam.
THIO is thiopental. L.S is Lycium schweinfurthii.
In Tables 3 and 4, the plant extract (400 mg/kg) significantly decreased the number of central, peripheral lines crossing and in the total lines crossing by 73.0%, 28.0% and 38.0%, respectively. There is no significant difference in the total rearing compared to the control group observed for four min. It produces a significant decrease in peripheral lines, total lines crossing and in total rearing by 64.0%, 61.0% and 71.0%, respectively, but did not produce a significant change in central lines crossing observed for 30 min as compared to the control group. The combination of plant extract (400 mg/kg) with diazepam (2 mg/kg) decreased the locomotor activity of the animals observed in 4 min and 30 min.
Groups/parameters |
Central activity |
Peripheral activity |
Total activity |
Total rearing |
Control |
11.33 ± 1.14 |
42.50 ± 2.15 |
53.83 ± 3.00 |
08.83 ± 0.91 |
L. S 400 mg/kg |
03.00 ± 0.81* |
30.50 ± 2.23* |
33.50 ± 1.83* |
05.67 ± 1.49 |
DZP 2 mg/kg |
15.83 ± 1.49*† |
96.50 ± 2.90*† |
112.33 ± 4.31*† |
17.50 ± 1.25*† |
L.S 400 mg + |
01.67 ± 0.49*‡ |
02.67 ± 0.91*†‡ |
4.67 ± 1.14*†‡ |
1.33 ± 0.33*†‡ |
DZP 2 mg/kg |
Table 3 Effect of Lycium schweinfurthii extract on exploratory activity of mice in 4 min
Data are mean ± SEM. *Significant difference from the control, †Significant difference from L.S extract and ‡Significant difference from DZP. DZP is diazepam and L.S is Lycium schweinfurthii.
Groups/ parameters |
Central activity |
Peripheral activity |
Total activity |
Total rearing |
Control 5 ml/kg |
19.17 ± 2.93 |
260.33 ± 6.27 |
279.50 ± 6.09 |
87.50 ± 5.38 |
L. S 400 mg/kg |
14.67 ± 1.96 |
93.17 ± 3.57* |
107.83 ± 4.00* |
25.00 ± 1.52* |
DZP 2 mg/kg |
70.83 ± 4.72*† |
353.00 ± 3.96*† |
423.83 ± 5.64*† |
178.17 ± 3.67*† |
LS 400 mg + |
03.00 ± 0.73*‡ |
30.00 ± 1.46*†‡ |
33.00 ± 1.12*†‡ |
04.50 ± 0.73*†‡ |
DZP 2 mg/kg |
Table 4 Effect of Lycium schweinfurthii extract on general locomotor activity of mice within 30 min
Data are mean ± SEM. *Significant difference from the control, †Significant difference from L.S extract and ‡Significant difference from DZP. DZP is diazepam. L.S is Lycium schweinfurthii.
Table 5 shows the effect of the central analgesic activity of the plant extract (400 mg/kg) in mice. Thus, an analysis of the data revealed a significantly increased in reaction time on a hot plate method as compared to the control group by 162.6%. However, mice treated with only diclofenac (50 mg/kg) produced significantly increased in reaction time compared to the control group by 128.3% but with no significant difference compared to the plant extract. When combined the plant extract with diclofenac, significantly increased by 425% in the reaction time compared to the control group and a significant change compared to the plant extract and diclofenac groups.
Groups |
Reaction time (sec) |
Control |
5.33 ± 0.42* |
L. S 400 mg/kg |
14.00 ± 0.57* |
diclofenac 50 mg/kg |
12.17 ± 1.13* |
L. S 400 mg + diclofenic 50 mg/kg |
28.00 ± 0.93*†‡ |
Table 5 Effect of Lycium schweinfurthii extract and diclofenac on hot plate test of analgesia
Data are mean ± SEM. *Significant difference from the control, †Significant difference from L.S extract and ‡Significant difference from diclofenac. L.S is Lycium schweinfurthii extract.
As shown in Table 6, indomethacin 20 mg/kg produced significantly increased in reaction time compared to the control group by 158.7% but, no significant change compared to the plant extract. However, the plant extract combined with indomethacin was significantly increased by 223% in reaction time compared to the control group with a significant difference compared to the plant extract and diclofenac groups.
Group |
Reaction time (sec) |
Control |
05.67 ± 0.42 |
L. S 400 mg/kg |
14.17 ± 0.60* |
Indomethacin 20 mg/kg |
14.67 ± 0.49* |
L. S 400 mg + Indomethacin 20 mg/kg |
18.33 ± 0.66*†‡ |
Table 6 Effect of Lycium schweinfurthii extract and indomethacin on hot plate test of analgesia
Data are mean ± SEM. *Significant difference from the control, †Significant difference from L.S extract
and ‡significant difference from indomethacin. L.S is Lycium schweinfurthii extract.
In table 7, the administration of the plant extract in a dose of 400 mg/kg produced significantly inhibition of writhing response by 81.5% compared with the control group. However, the effect of diclofenac (25 mg/kg) showed a significant reduction in writhing counts by 69.4% compared to the control group and a significant difference compared to the plant extract. Combination of plant extract with diclofenac produced a significant decrease in the number of writhing by 94.5% compared to the control group and a significant difference compared to the plant extract and diclofenac groups.
Groups |
Dose |
Number of writhing |
% Inhibition |
Control |
45.17 ± 1.35 |
- |
|
L. S |
400 mg/kg |
08.33 ± 1.08* |
81.55 |
Diclofenac |
25 mg/kg |
13.83 ± 0.47*† |
69.38 |
L. S + Diclofenac |
400 mg + 25 mg/kg |
02.50 ± 1.33*†‡ |
94.46 |
Table 7 Effect of Lycium schweinfurthii extract on acetic acid writhing response in mice
Data are mean ± SEM. *Significant difference from the control, †significant difference from L.S extract and ‡significant difference from diclofenac. L.S is Lycium schweinfurthii extract.
This study was designed to explore the effects of methanolic leaves extract of L. schweinfurthii on the primary observations of intact animals using the schedule screening methods described by Irwin.13 The finding of the Irwin test was used to determine the potential toxicity and to select doses for specific pharmacological activities. The present findings revealed that different doses of L. schweinfurthii extract showed a significant change in the parameters as alertness, passivity, spontaneous activity, visual placing, staggering gait, abnormal gait and palpebral opening. Moreover, a longer continuous observation for 48 hours revealed no mortality even with a very high dose (1600 mg/kg) of L. schweinfurthii. This indicates that the plant extract has a large safety of margin. To our knowledge, no previous published data support either sedative, hypnotic or general locomotor activities of this plant in animals or humans. Potentiation of benzodiazepine hypnosis was carried out in which diazepam was administered to mice pre-treated with L. schweinfurthii. The L. schweinfurthii administered does not induce sleep in mice but significantly prolonged the duration of sleep induced by diazepam. The ability of L. schweinfurthii leaves extract to potentiate the sedative property of diazepam suggests the presence of some components activating benzodiazepine and/or GABA receptors in the GABA receptor complex. Additionally to this, use of flumazenil antagonist of benzodiazepine receptor on the GABAA-benzodiazepine receptor complex24 which support this finding. Administration of flumazenil before L. schweinfurthii extract in diazepam-induced sleep did not loss of righting reflex. Thus, flumazenil completely blocked the hypnotic effects of the plant extract and diazepam. Further, the plant combination with thiopental did not show any significant effect in the onset and duration of sleep.
The general locomotor activity and the exploratory behaviour were measured by using open field test. The parameters were measured by the ambulatory activities by horizontal movements (the number of squares crossing in the central and peripheral lines and the exploratory activities by the vertical movements (rears in the central and peripheral),17 indicating the plant extract significantly decrease number of central, peripheral lines crossing and total lines crossing in the beginning of the test explaining a decrease in the exploratory activity, being the feature of sedation. Diazepam is a reference in this model produces a significant increase in the locomotor activity. On the other hand, the combination of the plant extract with diazepam produced a significant reduction in exploration and locomotion which are markedly observed for 4 min and 30 min. The effect of L. schweinfurthii extract on the general locomotor activity may support the previous results of the ability to increase the duration of sleep-in diazepam-induced sleep. The flavonoids have been determined the most content in the plant extract, which could play a pronounced effect on locomotor activity and sedative effect. The analgesic activity of the plant extract was investigated by thermal and chemical methods. Thus, with the peripheral analgesic activity, an analysis of the data revealed the plant extract reduced significantly the writhing response and a marked reduction in frequency of writhing in combination with diclofenac sodium to reach about 100%. The mechanism of acetic-acid induced writhing induction release of arachidonic acid via cyclooxygenase enzyme and prostaglandins synthesis.25 This result may be explained by the plant extract exerting it is analgesic effect probably by inhibition of prostaglandins synthesis. The marked central analgesic effect of the plant extract was determined to increase in reaction time on the hot plate method significantly. When given in combination with diclofenac, besides, the combination of the plant extract with indomethacin, a significant increase in reaction time on the hot plate. This indicates that the extract possesses the ability to reduce centrally mediated pain. Presently, qualitative phytochemical analysis of the methanolic leaves extract of L. schweinfurthii indicates the presence of flavonoids, tannins, saponins, phenols, cardiac glycoside, alkaloids and carbohydrates as a major secondary metabolites which might be responsible for the observed pharmacological activity. However, anthraquinone, resins and steroids are absent in this extract. As documented from the phytochemical method findings of a previous study carried out on Egyptian L. schweinfurthii leaves presented resins and steroids.3 The results of quantitative phytochemical analyses revealed that the presence of flavonoids which is the most content compound in the extract. So, it is a possible that flavonoids present in the extract may responsible for the pharmacological activity. The present study is not in line with the previous published data.3 This difference could be explained by geographic distribution and/or techniques used in both studies. However, a further study is needed to isolate the active compounds which responsible for the activities.
The present study concludes that L. schweinfurthii leaves methanolic extract possesses both sedative and potent central and peripheral analgesic activities and flavonoids may contribute to this activity.
The authors would like gratefully to thank Naeema M. Aljafry for her assistance.
The authors declare no conflict of interest.

©2022 Bazine, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.