eISSN: 2379-6367


Research Article Volume 6 Issue 6
Department of Pharmaceutical Chemistry, AIMST University, Malaysia
Correspondence: Naeem Hasan Khan, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, AIMST University, Bedong 08100, Kedah Darul Aman, Malaysia , Tel 0060 16 9372470
Received: November 18, 2018 | Published: December 18, 2018
Citation: Xia KZ, Perveen N, Khan NH. Phytochemical analysis, antibacterial and antioxidant activity determination of Ocimum sanctum. Pharm Pharmacol Int J. 2018;6(6):490-497. DOI: 10.15406/ppij.2018.06.00223
The objective was to analyse phytochemical constituents from the leaves of Ocimum sanctum using suitable solvents and extraction technique and to evaluate the in-vitro antibacterial and in-vitro antioxidant activities of leaf extract of Ocimum sanctum. In present work, Soxhlet extraction and maceration extractions were applied to the fresh leaves of the Ocimum sanctum by using absolute ethanol. Phytochemical analysis for the important chemical constituents from ethanolic extract was carried out. Antimicrobial activity of Ocimum sanctum extract was carried out using Well Diffusion method by comparing the clear inhibition zone of standard antibiotic and the extracts on the Mueller Hinton agar. Antioxidant activity of Ocimum sanctum was carried out performing total phenolic content test and DPPH to identify the percentage of scavenging by the chemical constituents. For phytochemical analysis, only test for alkaloids, test for terpenoids and test for carbohydrates showed positive results for Ocimum sanctum extract. For antibacterial screening, all the concentrations of OSESE showed negative results due to low concentration of extract being used. For antioxidant analysis, total phenolic contents and DPPH radical scavenging showed antioxidant result for OSESE. It is concluded that Ocimum sanctum is a very essential plant medicinally. A long term research project is a must to evaluate the pharmacological uses of extracts with different solvents that can be used to isolate the pure and high yield of chemical constituents from the plants.
Keywords: soxhelt, maceration, ethanol, phytochemical, antibacterial, anti-oxidant
The plant’s height is up to 1 m, with branches, carrying with a pungent aromatic odour smell, the branchlets and new growth pubescent with soft white hairs. The Ocimum sanctum’s leaves with blades elliptic to elliptic-oblong approximately 3 to 6cm long, width is1–2.5cm, cuneate to attenuate at base, obtuse to acute at apex, entire to remotely serrate at margins, pubescent on both surfaces but especially on the nerves beneath. For the flowers terminal, the slender racemes or panicles are 4 to 12cm long with the width of 1 to 1.5cm, the bracteoles are 2 to 3mm long, ovate, acuminate, ciliate; flowers in verticils, on the pedicels are 2 to 4.5mm long; at anthesis, the calyx c is 2.5mm long, in fruit up to 5mm long, glabrous within, the upper lip suborbicular, reflexed, short-apiculate, the lower lip longer than the upper lip, the teeth 4, lanceolate; corolla pale pink, pale lavender or white, to 4mm long; filaments of stamens exerted, slender, the upper pair of each with a small, bearded basal appendage. The appearance of fruit is purple-green to brown, broadly ellipsoid, approximately 0.8–1.2mm long, smooth to minutely pitted, swelling in water.1,2 The leaves and flowers of Ocimum sanctum are shown in Figure 1.
Other names of Ocimum sanctum are Tulsi, Tulasi, Gouri, Bhuteshta, Bhutaghini, Nagamata, Surasah, Mal-Tulasi, Krsiatulasi, Indian Basil, Holy Basil, Sacred Basil, Nalla Thulasi, Raihan, Lo-Le, Basil Icum, Basilic, Basilienkraut, Selasih, Kemangi, Basilico, Meboki, Selaseh, Belanoi, Sulasi, Man Jericao, Bazilik, Albahaca, Suwenda-Tala, Maduru-Tala, Basilkort, Horopa, Manghk, Krapow, Bai Horapa, Rau Que.3,4 It is available in India, Sri Lanka, Himalaya, Bangladesh, South West Asia, Burma, China, Thailand, Malaysia. In addition, it is also available at dry sandy areas in Hainan, Sichuan, Taiwan Cambodia, Indonesia, Laos, Myanmar, Philippines, Vietnam; Africa, South West Asia, Australia.2 Ocimum sanctum, the Queen of medicinal herbs is the holiest and the most valuable of the many healing and ill-health giving herbs of the suitable way.5,6 The blessed Basil or Tulsi is significant in the traditional Ayurvedic and Unani system.5 In India, Ocimum sanctum are believed that it can be given the treatment of bronchitis, bronchial asthma, malaria, diarrhoea, dysentery, skin diseases, arthritis, painful eye diseases, increase in body temperature and also insect bite. More importantly it has anticancer, antifungal, antihypergycaemic, antibacterial, in treatment of nausea and vomiting, protection against liver and heart, analgesic, adaptogenic and diaphoretic actions. It improves the body immune system, reproductive system, CNS, CVS, gastrointestinal tract system, urinary system and also blood circulation.14 Table 1 show the nutritional facts about Ocimum sanctum.
In fresh Tulsi, five leaves were tested. (2.5g) |
|
Calories |
0.675 |
Protein |
0.064g |
Carbohydrate |
0.108g |
Total Fat |
0.015g |
Fiber |
0.098g |
Table 1 Nutritional highlight of Ocimum sanctum
Collection and preparation of plant materials
Green and fresh 557g of Ocimum sanctum was collected from the plants. The leaves were cleaned by distilled water and then leaves were separated from the branches manually. The separated leaves were weighed again and net weight was 349.64g and allowed for air drying under the room temperature to avoid destruction of active group in the leaves. The dried leaves were crushed by using hand into very small pieces.
Maceration
The 25.0g of crushed raw material was subjected to maceration with 200ml of absolute ethanol in round bottom flask and sealed with the aluminium foil and kept in the dark for seven days. The round bottom flask was shaken throughout to ensure uniform and complete extraction. The mixture was filtered by using clean Muslin cloth and the filtrate was collected in a cleaned beaker. The residue of maceration extract and filtrate of maceration were separated and being kept inside the cabinet for further screening.
Soxhlet extraction
26.0g of the crushed powder form was placed inside a thimble already fixed with the chromatographic paper. Ethanol added was 350ml for the extraction and poured into the round bottom flask of Soxhlet apparatus. The temperature was kept at 70°C and maintained throughout the process. The whole process took about 30 hours to complete till the clearance of colour extract. The residue of maceration extract and filtrate of maceration were separated and being kept inside the cabinet for further screening.
Evaporation
The evaporation was carried out from the extract (Soxhlet and Maceration) in a rotary evaporator. The temperature was set at 70°C throughout the evaporation and concentration. The extracts of Soxhlet and maceration, after evaporation, were 88ml and 25ml respectively.
The results of phytochemical analysis is recorded and tabulated in Table 2.7–12
No. |
Phytochemical Tests |
Ethanolic maceration |
Ethanolic Soxhlet Sample |
|
|
|
Filtrate |
Residue |
|
1 |
Alkaloids |
+ |
+ |
+ |
2 |
Reducing Sugar |
- |
- |
- |
3 |
Saponins |
- |
- |
- |
4 |
Terpenoids |
+ |
+ |
+ |
5 |
Antraquinones |
- |
- |
- |
6 |
Glycosides |
- |
- |
- |
7 |
Tannins |
- |
- |
- |
8 |
Flavonoids |
- |
- |
- |
9 |
Carbohydrate |
+ |
+ |
+ |
Table 2 Results for qualitative phytochemical screening
+ presence, - absence
Methodology for determination of antibacterial activity Preparation of Luria Bertani broth media
2.0g of Luria Bertani broth was dissolved in 100ml distilled water. Then, 10ml of Luria Bertani broth was poured into each 4 universal bottles and subjected to the autoclave at high pressure saturated steam 121°C for around 1 hour in the laboratory.13–17
Preparation of Muller Hinton Broth
38.0g Muller Hinton Broth was dissolved into 1000ml of distilled water and poured into two 500ml of Scott bottles. After that, the two Scott bottles were taken to autoclave at high pressure saturated steam 121°C for around 1 hour in the Biotechnology laboratory. After the autoclaving have done, the sufficient sterilized quantity of Muller Hinton agar was poured into the sterilized petri plates and was allowed to solidify. Agar plates were stored in incubator at about 37°C.13–17
Bacteria strains cultures
Bacteria strains of Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli and Streptococcus pyogenes were cultured using Luria Bertani broth in 4 different universal bottles. The bacterial strains stored in the universal bottles were left shaking incubator for 24 hours at 37°C at 180rpm. The next days, the bacteria strains which has grown in Luria Bertani broth is then cultured into Muller Hinton agar.16
Dilution of extracts16
(dissolved in sterilized distilled water)
Well Diffusion test preparation in laminar air flow cabinet16,17
Result for the Well Diffusion Test. Table 3 indicates the zone of inhibition in mm. In present antibacterial study, two different extracts with three different concentrations were investigated to detect the zone of inhibition against Bacillus subtilis, Pseudomonas aeruginosa, Streptococcus pyogenes and Escherichia coli. Ciprofloxacin was used as a positive control and sterilized distilled water was used as a negative control. As a result, there is no antibacterial inhibition zone occurred in the most of the agar plates. This may be due to low concentration of the extract being used. Low concentration of the Ocimum sanctum was unable to produce any antibacterial effect against the bacteria strains in the agar plate. Figure 2, Figure 4, Figure 6 and Figure 8 shows the ethanolic maceration extract and Figure 3, Figure 5, Figure 7 & Figure 9 indicates the ethanolic Soxhlet extract on the agar plate. All Figures shown at lower region, from 2–9, showed that the agar plates do not have any antibacterial activity by well diffusion method. There was an absence of zone of inhibition for each of the different concentration used by the extract solution.16,17
Microorganisms |
Concentrated extract |
1 mg/ml |
5 mg/ml |
10 mg/ml |
Ciprofloxacin |
Sterilized distilled water |
Bacillus subtilis |
Ethanolic Maceration |
- |
- |
- |
24 |
- |
Ethanolic Soxhlet |
- |
- |
- |
24 |
- |
|
Pseudomonas aeruginosa |
Ethanolic Maceration |
- |
- |
- |
26 |
- |
Ethanolic Soxhlet |
- |
- |
- |
24 |
- |
|
Streptococcus pyogenes |
Ethanolic Maceration |
- |
- |
- |
26 |
- |
Ethanolic Soxhlet |
- |
- |
- |
26 |
- |
|
Escherichia coli |
Ethanolic Maceration |
- |
- |
- |
24 |
- |
Ethanolic Soxhlet |
- |
- |
- |
24 |
- |
Table 3 Zone of inhibition in mm
Pseudomonas aeruginosa
Escherichia coli
Bacillus subtilis
Streptococcus pyogenes
All Figures from 2–9 showed that the agar plates do not have any antibacterial activity determination by well diffusion method. There was an absence of zone of inhibition for each of the different concentration used by the extract solution.
Antioxidant activity determination
Methodology for determination of antioxidant activity
Total phenolic contents analysis (TPC)18: For the total phenolic contents analysis, Folin-Ciocalteau (FC) assay was applied to Ocimum sanctum ethanolic Soxhlet extract to find out the total phenolic content of this extract. In this analysis, the standard used was Gallic acid and the measurement was done at λ max 765nm. Gallic acid standard calibration curve was generated as shown in Graph 1 and the regression value was 0.9884. From the absorbance obtained, the amount of the phenolic content measured as Gallic acid equivalent (GAE) using FC method. The total phenolic content obtained at the concentration of 1.0mg/ml was found to be the highest when compared to other lower concentration extract solution. As a result, the higher the concentration of the stock solution used for this analysis, the higher the Gallic acid equivalent (GAE).19–25
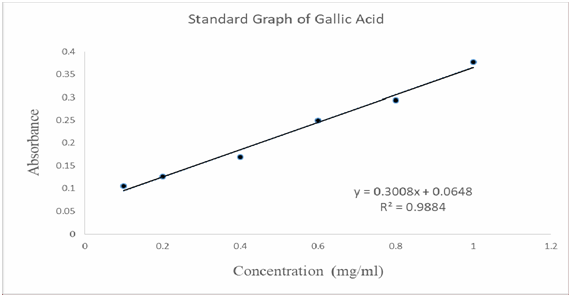
Graph 1 Standard graph of the Gallic acid plotted from the series of absorbance obtained from UV- Visible Spectrophotometer.
Extract preparation
Preparation of sample extract, blank and standard16,17
C= (A/B) x dilution factor
C, Total phenolic content; A, x value; x, regression line; B, concentration of extract
DPPH free radical scavenging assay16
Results of antioxidant activity
The results are shown in Table 4, Table 5, Table 6.
OSESE
Concentration |
y |
x |
A |
B |
Dilution factor |
C |
0.1mg/ml |
0.3008 x + |
0.073mg/ml |
0.073mg/ml |
0.1mg/ml=0.0001g/ml |
1/10000 |
(A/B) x dilution factor= (0.073/0.0001) x 1/10000= 0.073 mg GAE/g |
0.0648 |
||||||
0.067= |
||||||
0.0648 |
||||||
0.3008 x = |
||||||
0.0022 |
||||||
0.2mg/ml |
0.3008x + |
0.084mg/ml |
0.084mg/ml |
0.2mg/ml=0.0002g/ml |
1/5000 |
(A/B) x dilution factor= (0.084/0.0002) x 1/5000= 0.084 mg GAE/g |
0.0648 |
||||||
0.090 = |
||||||
0.3008x + |
||||||
0.0648 |
||||||
0.3008x = |
||||||
0.0252 |
||||||
0.4mg/ml |
0.3008x + |
0.297mg/ml |
0.297mg/ml |
0.4mg/ml=0.0004g/ml |
1/2500 |
(A/B) x dilution factor= (0.297/0.0004) x 1/2500= 0.297 mg GAE/g |
0.0648 |
||||||
0.154 = |
||||||
0.3008x + |
||||||
0.0648 |
||||||
0.3008x = |
||||||
0.0892 |
||||||
0.6mg/ml |
0.3008x + |
0.513mg/ml |
0.513mg/ml |
0.6mg/ml=0.0006g/ml |
3/5000 |
(A/B) x dilution factor= (0.513/0.0006) x 3/5000= 0.513 mg GAE/g |
0.0648 |
||||||
0.219 = |
||||||
0.3008x + |
||||||
0.0648 |
||||||
0.3008x = |
||||||
0.1542 |
||||||
0.8mg/ml |
0.3008x + |
0.679mg/ml |
0.679mg/ml |
0.8mg/ml=0.0008g/ml |
1/1250 |
(A/B) x dilution factor= (0.679/0.0008) x 1/1250= 0.679 mg GAE/g |
0.0648 |
||||||
0.269 = |
||||||
0.3008x + |
||||||
0.0648 |
||||||
0.3008x = |
||||||
0.2042 |
||||||
1.0mg/ml |
0.3008x + |
0.961mg/ml |
0.961mg/ml |
1.0mg/ml=0.001g/ml |
1/1250 |
(A/B) x dilution factor= (0.961/0.001) x 1/1000= 0.961 mg GAE/g |
0.0648 |
||||||
0.354 = |
||||||
0.3008x + |
||||||
0.0648 |
||||||
0.3008x = |
||||||
0.2892 |
|
|
|
|
|
|
Table 4
S. No |
Concentration of Gallic acid / mg/ml |
Absorbance |
1 |
0.1 |
0.105 |
2 |
0.2 |
0.126 |
3 |
0.4 |
0.169 |
4 |
0.6 |
0.25 |
5 |
0.8 |
0.294 |
6 |
1 |
0.377 |
Table 5 UV absorbance of Gallic acid in various concentration
Conc. Of Sample (mg/mL) |
100 |
10 |
1 |
0.1 |
0.2 |
0.3 |
0.4 |
0.6 |
0.8 |
Stock solution (ml) |
1000 |
100 |
10 |
1 |
1 |
1 |
1 |
1 |
1 |
Vol. of stock solution (ml) |
1 |
1 |
1 |
1 |
2 |
3 |
4 |
6 |
8 |
Vol. of 95% methanol (ml) |
9 |
9 |
9 |
9 |
8 |
7 |
6 |
4 |
2 |
Total Volume (ml) |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
Dilution factor |
1/10n |
1/100 |
1/1000 |
1/10000 |
1/5000 |
3/10000 |
1/2500 |
3/5000 |
1/1250 |
Table 6 Dilution factors for Gallic acid in total phenolic analysis
DPPH free radical scavenging analysis18
Concentration (mg/ml) |
UV Absorbance |
|
BHT |
OSESE |
|
0.03125 |
0.442 |
0.468 |
0.0625 |
0.32 |
0.421 |
0.125 |
0.233 |
0.314 |
0.25 |
0.102 |
0.168 |
0.5 |
0.03 |
0.053 |
1 |
0.02 |
0.038 |
Table 7 Absorbance value of standard (BHT) and extract of Ocimum sanctum at 517nm, Control (DPPH) absorbance = 0.550
DPPH ASSAY |
||
Concentration (mg/ml) |
%Scavenging |
|
BHT |
OSESE |
|
0.03125 |
19.64 |
14.91 |
0.0625 |
41.82 |
23.45 |
0.125 |
57.64 |
42.91 |
0.25 |
81.45 |
69.45 |
0.5 |
94.55 |
90.36 |
1 |
96.36 |
93.09 |
IC50 |
0.1 |
0.25 |
Table 8 Percentage scavenging of BHT and OSESE in DPPH assay
Graph 4 shows a gradually decrease in the scavenging ability of the OSESE sample to the standard BHT. OSESE shows IC50 of 0.25mg/ml which was comparable to BHT with IC50 of 0.10mg/ml. The IC50 of BHT was calculated by the equation which plotted from the standard graph of BHT as shown in Graph 2 and the IC50 of OSESE was calculated by the equation plotted from the graph of concentration against % scavenging of OSESE as shown in Graph 3.
For BHT:
IC50 |
Y = MX + C |
50 = 66.936x + 43.279 |
66.936x = 50 - 43.279 |
66.936x = 6.721 |
x = 6.721/66.936 |
x = 0.1mg/ml |
For OSESE:
IC50 |
Y = MX + C x = 19.749/77.551 |
In DPPH scavenging method, the result obtained for OSESE at 1.0mg/ml stock concentration was 93.09mg/ml whereas in total phenolic content analysis, the result obtained for OSESE at 1.0mg/ml stock concentration was 0.961mg GAE/g.
Hence, this can be proved that this extract has the antioxidant potential. The preliminary in- vitro antibacterial screening of Ocimum sanctum was not effectively showed any control over the growth of the test bacteria strains due to low concentration used in this study. To have more insight into the antibacterial screening, further investigation on isolates of extracts should be done perfectly. Ocimum sanctum has shown substantial antioxidant activity through total phenolic content analysis and DPPH radical scavenging analysis. It is concluded that there is a good antioxidant potential of Ocimum sanctum with ethanolic Soxhlet extraction.
The authors are grateful to the Faculty of Pharmacy, AIMST University, Bedong, Kedah D.A., Malaysia for funding and providing entire research facilities.
None.
Authors declare that there is no conflict of interest.

©2018 Xia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.