eISSN: 2379-6367


Research Article Volume 7 Issue 2
1Department of Pharmacology and Traditional Medicine, Faculty of Medicine and Biomedical Sciences, Yaounde I University, Cameroon
2Universite des Montagnes, Cameroon
3National Drug Quality Control and Valuation Laboratory, Cameroon
4Departement de Pharmaco chimie Moleculaire, Universite de Grenoble Alpes, France
5Department of Therapeutic Chemistry and Pharmacognosy, Faculty of Medicine and Biomedical Sciences, Yaounde I University, Cameroon
6Plantes Medicinales Heritage Africain, Cameroon
Correspondence: Ngono Mballa Rose, Department of Pharmacology and Traditional Medicine, Faculty of Medicine and Biomedical Sciences, Yaounde I University, Cameroon
Received: March 27, 2019 | Published: April 9, 2019
Citation: Rose NM, Noel WM, Denis W, et al. Pharmacognosic standardization of a traditional recipe for incurable wounds based on Petersianthus macrocarpus (Lecythidaceae), Vernonia conferta (Asteraceae), and Carica papaya (Caricaceae) in Cameroon . Pharm Pharmacol Int J. 2019;7(2):86-91. DOI: 10.15406/ppij.2019.07.00236
Standardization is a process that allows botanical, chemical, pharmacognosic or even pharmacological characterization of herbal medicines. This operation will consist of a macroscopic and microscopic examination of the medicinal material of the recipe (remedy’s mixture of powders). Then, a phyto chemical screening of the extract prepared in accordance with the practices of the healer will be carried out. Finally, the desiccation loss and the total ash content will be determined. The recipe used for incurable wounds was obtained following an ethno botanical survey carried out with a traditional healer. Indeed, traditional medicine continues to provide therapeutic solutions through plants. Chronic wounds are severe affections by their manifestations. Their care is difficult because of the multitude of factors behind their constitution. Many conventional therapies are available but mostly unaffordable and less and less effective. The goal of this work was to develop a new affordable and effective treatment. Our work has therefore successfully standardized a traditional recipe based on Petersianthus macrocarpus (Lecythidaceae), Vernonia conferta (Asteraceae), and Carica papaya (Caricaceae), used locally in the treatment of chronic wounds. This research is part of the program initiated by Abondo-Ngono Mballa et al.,1 on cartography of Healers from the Center Region Cameroon, followed by Ngono Mballa et al.,2 and Minyem et al.,3 in standardization of a recipe traditionally used to cure scalp fungal dermatitis. The standardization approach is therefore the appropriate solution to identify chemical and biochemical markers, constituting the substances and compounds of the plants present in the recipe and which would be responsible for the therapeutic activity.
Keywords: chronic wounds, traditional medicine, standardization, bark
Wounds are break-ins of the cutaneous barrier. They fall into two broad categories: acute wounds and chronic wounds.4 The chronic wounds are characterized by an extended healing time (4 to 6 weeks of evolution, depending on the etiology).5 Their prevalence in the general population is estimated at 1% and their prevalence in the population over 65 years of age, is at up to 3 to 5%.6 In a context of low affordability in access to expensive care, combined with the resistance of microorganisms to anti-infectives, exploring the vast reservoir of natural resources like medicinal plants is a preferred pathway.7 In fact, and in general in Africa, about 80% of the population uses traditional medicine.8 In addition, traditional healers have always been the primary caregivers of many patients with limited resources, and some of them are well recognized in their respective communities. Therefore it is necessary to search in the therapeutic heritage of our recipes/traditional remedies, with a view to standardize these very products, in order to ameliorate the quality. Standardize means implement quality procedures at all stages of manufacture, from the crude drug to the extract, to achieve a specified known or required standard. All the parameters influencing the quality of the product (extract, finished product) must be defined, followed and applied from the raw material (its origin, the cultivation methods, the concern part of the plant, its identity, its purity, its active principle content), but also the nature and concentration of the extraction solvent, as well as the manufacturing process (maceration/percolation, temperature, time, pressure during manufacture, drying process, controls during manufacture).9 Standardization therefore, consists in ensuring a constant pharmaceutical quality for a herbal medicine. A study conducted in the Center region had made possible, to establish an almost exhaustive list of recipes, used by many traditional healers to treat several types of affections.1 Then, we decided to standardize a traditional recipe for the treatment of chronic wounds, found in the above mentioned document, which was made up by three plants: Petersianthus macrocarpus (Lecythidaceae), Vernonia conferta (Asteraceae), and Carica papaya (Caricaceae).
Vegetable equipment
It was harvested in February 2017 in the Central Region in Mbankomo, department of Méfou-et-Akono. The identification was made at the National Herbarium of Cameroon. The stem bark of Petianthus macrocarpus was identified by comparison with the sample of Endengle Elias No. 187 (19089/HNC), collected on 07/03/1996. The trunk bark of Vernonia conferta was identified by comparison with the Duncan W. Thomas sample No. 6847 (55447/HNC), collected on 25/03/1987. Carica papaya root barks was identified by comparison with the ECOFAC sample No. 999 (66220/HNC), collected on 08/09/1995. After the macroscopic analysis, the barks were dried-shading during a week and then, grounded with an electric crushing mill.
Macroscopic examination10
Whole barks were examined and manipulated to measure the thickness, to identify the color, the texture, the degree of fragility, the characteristics of fissures, and finally the strength and odour feeling.
Microscopic examination11
In a lab recipient, powders were wetted firstly with distilled water, and secondly with a 5% w/v aqueous solution of sodium hydroxide (with a view to completely or partially destroying certain elements as plant cells, may be include or not in parenchymal cells, in order to rend cell walls sharper and easier to observe, specially the sclerified elements, fibers and cuticles). Samples were then observed under the optical microscope CAMERA IRMECO®.
Pharmacognosic standardization of recipe (Traditional Remedy)
It involved carrying out testing to characterize the components present in the recipe and which may be responsible for its activity, i.e a phytochemical screening.12 Then we performed two quality control testing’s of plant materials, loss on drying and total ash content, according to WHO Guidelines.10
Extraction
To 75g of powder we added 150ml of distilled water and brought the mixture to boil during 15 minutes. The filtrate obtained was reduced by evaporation, to obtain a weight equal to the mass of initial plant material (fluid extract).
Search for flavonoids
To an aliquot of decoction, we added few drops of ethanol (C2H5OH), hydrochloric acid (HCl) and some magnesium slices. The appearance of pink-orange or purplish color was an indication of positive reaction.
Search for alkaloids
In two test tubes, an aliquot acidified decoction with hydrochloric acid was distributed; we add few drops of Dragendorff’s reagent in one, and in the other, drops of Mayer reagent. The appearance of orange-red precipitate or a ladle was an indication of positive test.
Search for tannins
To an aliquot decoction we add 15ml of Stiasny's reagent. The presence of a brown precipitate was an indication of catechic tannins presence. On the filtrate of the previous mixture, we add few drops of iron chloride III (FeCl3) at 2%. The appearance of a blue-black color indicated the gallic tannins presence.
Search for saponosides
We introduced 5ml of decoction into a test tube and stirred vigorously for 2min. The formation of a foam (height greater than 1 cm) stable, persisting in 15 minutes indicated saponins presence.
Search for steroids and terpenoids
To an aliquot decoction, we added 1ml of acetic anhydrous (C4H6O3) and 0.5ml of concentrated sulfuric acid (H2SO4). The appearance of a purple color that turns blue then green indicated the presence of sterols, while the appearance of a red ring indicated the presence of polyterpens.
Search for coumarines
To an aliquot decoction, we added ethanol and then divided into two test tubes. To one, we added 0.5ml of 10% sodium hydroxide (NaOH), and then the tubes were heated in a water bath until boiling. After cooling, we added in each tube, 4ml of distilled water and few drops of concentrated hydrochloric acid. The formation of a precipitate in the tube containing the mixture indicated a positive reaction.
Search for bound quinones
To an aliquot decoction, we added 5ml of hydrochloric acid diluted to 1/5 and heated all in boiling water bath for 30min. After cooling, the organic phase was extracted with 20ml of chloroform (CHCl3) and a 50% dilution of ammonia (NH4OH) was added. The appearance of a hue ranging from red to purple indicated a positive reaction.
Loss on drying
5g of powder were deposited in a beaker previously dried, weighed and tarred, then heated in an oven at 105°C during 24 hours. The percentage of loss on drying was calculated according to the amount of initial powder.
Total ash
2g of powder were introduced into a crucible previously heated, weighed and calibrated, then heated to 500-600°C, until the ashes become white. The total ash content was calculated based on the amount of initial powder.
Macroscopic examination
The different observations are mentioned in Table 1.
Microscopic examination
Extraction
The different extraction yields, calculated for the aqueous extracts and the hydro-alcoholic extracts, are listed in Table 2.
Phytochemical screening
The different secondary metabolites found are listed in Table 3.
Analysis of the phytochemical composition revealed the presence of alkaloids, catechic tannins and terpenoids in the trunk bark of Vernonia conferta, tannins, saponosides, terpenoids and bound quinones in the bark of Petersianthus macrocarpus trunk, and terpenoids in the root bark of Carica papaya.
Physicochemical testing’s
The percent losses of desiccation and total ash measured are shown in Table 4 below.
|
|
Trunk bark of Vernonia conferta |
Trunk bark of Petersianthus macrocarpus |
Root bark of Carica papaya |
|
Dimensions (mm) |
3-5* |
4-7* |
8-220** |
|
External color |
Black green |
Brown |
Milky |
|
Internal color |
Dark brown |
Brown |
White |
|
Texture |
Tough |
Tough |
Fresh |
|
Fissure plan |
Fibrous |
Fibrous |
Smooth |
|
Smell of the powder |
Distinct, acrid |
Weak |
Distinct, fruity and spicy |
Table 1 Macroscopic physical characteristics of cark
*Thickness **Diameter
|
Initial powder masses |
Masses of extracts obtained |
Yield |
|
|
Vernonia conferta (100g) |
Aqueous extract |
5g |
5% |
|
Hydro-alcoholic extract |
4.5g |
4.5% |
|
|
Petersianthus macrocarpus (100g) |
Aqueous extract |
9.3g |
9.3% |
|
Hydro-alcoholic extract |
11.4g |
11.4% |
|
|
Carica papaya (100g) |
Aqueous extract |
6g |
6% |
|
Hydro-alcoholic extract |
4.7g |
4.7% |
|
Table 2 Extraction yields
|
|
Trunk bark of Vernonia conferta |
Trunk bark of Petersianthus macrocarpus |
Root bark of Carica papaya |
|
alkaloids |
+ |
- |
- |
|
Catechic tannins |
+ |
++ |
- |
|
Gallic tannins |
- |
+ |
- |
|
saponosides |
- |
+++ |
- |
|
flavonoids |
- |
- |
- |
|
Terpenoids |
++ |
+ |
+ |
|
Steroids |
- |
- |
- |
|
Coumarins |
- |
- |
- |
|
Linked quinones |
- |
++ |
- |
Table 3 Phytochemical compositions
+: weakly present; ++: medium present; +++: highly present; -: absent
|
|
Vernonia conferta |
Petersianthus macrocarpus |
Carica papaya |
|
Loss on drying (%) |
9.33 |
8.67 |
42.67 |
|
Total ash (%) |
7 |
5.2 |
11 |
Table 4 Physico-chemical tests
After an ethnobotanical survey done by the traditional healer who conducted in harvesting plants, we carried out various testing on samples, in order to develop plant monographs. After a complete macroscopic analysis, the plants were crushed for microscopic analysis. The micrograph of Carica papaya (Figure 1) revealed the presence of calcium oxalate crystals and palissadic tissues, which is a conductive tissue. Roy et al.,13 also found on trunk powder of Carica papaya, calcium oxalate crystals, with parenchymal cells and several conductive tissues. The analysis of Vernonia conferta powder (Figure 2) showed the presence of support tissues (collenchyme, suber, epidermis fragment covered with hair like outgrowth from the surface, sclerified fibers), conductive tissues (bast fibers), perimedullary tissue and calcium oxalate crystals. Evans et al.,14 had observed irregular epidermal cells, anomocytic stomata, and T-shaped glandular hair like outgrowth from the surface. Singh et al.,15 Vernonia cinerea bark cells, parenchymatous cells, hair like outgrowth from the surface, fibers and vessels were found on the powder of Vernonia cinerea bark. The micrograph of the bark powder of Petersianthus macrocarpus (Figure 3) showed the presence of secondary xylem, a mass of conductive tissue, fibers of the palissadic tissue and sclerotic cells. Lens et al.,16 had observed inter alia axial parenchymal cells.

Figure 1 Micrograph of fresh roots of Carica papaya: observation with sodium hydroxide. (A) Calcium oxalate crystals. (B) Palisade tissue structure (conductive tissue) (Source: present study).
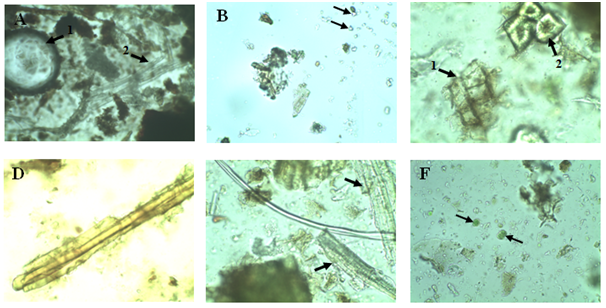
Figure 2 Micrograph of the powder of dry bark of Vernonia conferta: observation with water. (A) 1. Conductive tissues. 2. Cluster of Liberian fibers. (B) Calcium oxalate crystals. (C) 1. Suber. 2. Collenchyme. (D) Fragment of epidermis covered with toothbrushes. (E) Sclerotized fibers. (F) Primary Perimedullary Screened Tissues (Source: present study).
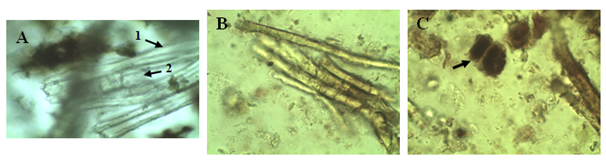
Figure 3 Micrograph of the powder of the dry bark of Petersianthus macrocarpus: observation with water. (A) 1. Conductive tissues. 2. Secondary Xylem. (B) Fibers of the palissadic tissue. (C) Sclerous cells. (Source: present study).
The various powders were then extracted using 02 solvent systems: water and an ethanol-water mixture (70/30, v/v). We obtained relatively high extraction yields, which are very important because it facilitates the valorization of the recipe: it was proven that the medical material will always be sufficient for the manufacture of improved traditional medicines. The subsequent phytochemical screening revealed the presence of few secondary metabolites, mainly alkaloids, tannins, saponosides, terpenoids and bound quinones (Table 3). These metabolites play an important role in the wound healing process by an amazing virtue of analgesic (alkaloids), antibacterial (tannins), and detersive, anti-inflammatory and antifungal properties (saponosides).17
Previous results from screening done by Kunle et al.,17 on various extracts of plants from the genus Vernonia reinforce our knowledge in the richness of this group in alkaloids, flavonoids, steroids and terpenoids. N'guessan et al.,18 found in extracts of Petersianthus macrocarpus, large amounts of polyphenols, flavonoids, catechic tannins, alkaloids and saponosides. Mabeku et al.,19 found a similar result and also sterols. Verma et al.,20 found in the aqueous extract of dry roots of Carica papaya tannins, sterols and cardiotonic glycosides, while Yusha et al.,21 observed tannins and saponosides in the aqueous extract of dry leaves. These differences in composition could be explained by the location and the period of the harvest, as well as by the nature of the plant part analyzed.
Physicochemical testing’s carried out on these plants allow us to determine for each, the percentages of loss on desiccation and total ash (Table 4). We obtained for Vernonia bark powder a loss on drying of 9.33%, a value close to that was obtained by Kunle et al.,17 on the entire plant of Vernonia ambigua (8.20%); the total ash content is 7%, similar to that of Singh et al.,15 for the trunk bark of Vernonia cinerea (6.08±1%). For Petersianthus macrocarpus bark powder, results were 8.67% for loss on drying and 5.2% for total ash. Satish et al.,22 obtained different significantly values, 12% and 14% respectively for the barks of Careya arborea. This difference is probably due to the fact that the 02 species which belong to the same subfamily are from different genus. The specificities of the bark will not necessarily be the same. We obtained for the fresh roots of Carica papaya a loss on drying of 42.67% and a total ash content of 11%. Roy et al.,13 obtained a total ash content for the trunk bark of Carica papaya (10%): the rather high value of the water content can be explained by the fresh state of the plant sample.
This work is part of the valorization of research on medicinal plants, traditional remedies and the development of Traditional ameliorated Medicines, from Traditional knowledge, through the standardization of recipes, in use by traditional healers within long generations. Minyem, moreover, has done a similar job relying on a local herbal recipe traditionally used to treat scalp fungal dermatitis.23 After a pharmacognosic analysis as complete as possible, we were able to set up a monograph of each of the plants, giving information on their macroscopic characteristics, their microscopic structures, their chemical composition, as well as their water and total ash contents according to the European Pharmacopoeia. Phytochemical screening revealed the presence of alkaloids, tannins, saponosides, terpenoids and quinones. These metabolites play a role in the healing process of wounds through their analgesic (alkaloids), antibacterial (tannins), detersive, anti-inflammatory and antifungal (saponoside) properties. In addition, terpenoids which are principles of essential oils, presents in these plants, act by their antiseptic power against various
We would like to thank Pr Denis WOUESSIDJEWE and Dr. Rose NGONO MBALLA for the supervision and contribution in the development of this publication. Our thanks also go to Prof. Joseph NGOUPAYO, Dr Aude Perrine MINYEM, and Victorine BEYENE for the participation and follow-up in this study. We will not fail to thank the Laboratory of Pharmacognosy of the University of the Mountains and also the Cameroon National Drug Quality Control and Valuation Laboratory (LANACOME), for the technical platform.
Authors declare that there is no conflict of interest.

©2019 Rose, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.