eISSN: 2379-6367


Opinion Volume 5 Issue 6
IFBV-BELHERB, Luxembourg
Correspondence: Pierre Lutgen, IFBV-BELHERB, BP 98 L-6905, Niederanven, Luxembourg
Received: November 09, 2017 | Published: December 6, 2017
Citation: Lutgen P. New insights into malaria prophylaxis. Pharm Pharmacol Int J. 2017;5(6):213-217. DOI: 10.15406/ppij.2017.05.00141
So far all efforts of the pharmaceutical industry were vain to find a drug with prophylactic properties against malaria. Artemisinine and chloroquine only have an effect during the intraerythrocytic stage. Other monotherapies have severe side effects for some categories of the population. In the most favorable vaccine clinical trial only 30% efficiency has been demonstrated. But since generations traditional healers apply treatments which are prophylactic. Most of these are based on medicinal plants, like for example Artemisia afra. The purpose of this paper is to review existing know-how on some of the molecules or minerals playing a role in these polytherapies: taurine, potassium, vitamin E, fats, sulfur.
The clinical assays of Jerome Munyangi in RDC1,2 following those of Patrick Ogwang in Uganda3 have clearly shown that Artemisia annua has prophylactic properties against malaria, but it is not known how and why. Artemisia plants are rich in polyunsaturated fatty acids (PUFA) which generate prostaglandins and stimulate monocytes. PUFAs possess well documented antimalarial and prophylactic properties. Their half-life in plasma is several days and in adipose tissue several weeks.4
Fats
The positive effect of a ketogenic, high fat diet on malaria is known since 70years. In a trial in India, of 10 rats, 8 weeks old, 5 received a standard diet and 5 a ketogenic diet containing 93% percent, butter. After a week, all were inoculated with Plasmodium berghei. The number of parasites observed daily and at the peak of infection was much less in the rats given the ketogenic diet.5 More recently a paper from Portugal has shown that administration of a high-fat diet to mice for a period as short as 4days impairs Plasmodium liver infection by over 90%. Plasmodium sporozoites can successfully invade and initiate replication but die inside hepatocytes, thereby are unable to cause severe disease. Transcriptional analyses combined with genetic and chemical approaches reveal that this impairment of infection is mediated by oxidative stress. Reactive oxygen species, probably spawned from fatty acid β-oxidation, directly impact Plasmodium survival inside hepatocytes, and parasite load can be rescued by exogenous administration of antioxidants. Together, these data reveal that acute and transient dietary alterations markedly impact the establishment of a Plasmodium infection and disease outcome.6
Taurine
Only animal fats like butter have this prophylactic effect, palm oil for example not. Taurine, a sulfur containing amino acid is the most abundant amino acid in mammals, it is absent in plants. Taurine homeostatis which occurs in the liver appears to be essential in this mammal for resistance to malaria. It is claimed that because of their ketogenic diet rich in meat and rich in taurine, cats and dogs are efficiently protected against malaria.7 Taurine has anti-oxidant properties.8 Upon infection by Plasmodium, the time course for the uptake into malaria-infected erythrocytes of taurine is strongly enhanced. In vitro, an intracellular concentration similar to the extracellular concentration is reached approximately within 10minutes. By contrast, there is little if any uptake of taurine by uninfected human erythrocytes over the same period.9 Taurine reduces accumulation of lipids in the liver and is protective against hepatic steatosis, more particularly palmitic acid, the most common saturated fatty acid found in animals and plants.10 Taurine has a strong effect on immunity. Replacement 50% of the sulfated amino acid methionine, mainly of plant origin, by taurine doubles IgA in broilers and increases IgM by 50%.11 The mystery of the invasion of hepatocytes through Kupffer cells may eventually find an answer in this context. Kupffer cells are specialized macrophages and protect the liver against microbes, contaminants and other aggressions. Why these phagocytes are used as entry gate by sporozoites indeed is difficult to understand.12 But some early studies have shown that IgA antibodies preferentially attach to hepatocytes, blocking the entry for sporozoites. Their number on the surface of Kupffer cells is much lower, 10% versus 63% on hepatocytes almost an open door.13 If so it is logical to expect that taurine has prophylactic antimalarial properties. In mice, the circulating taurine is around 550µmol/liter. If this concentration falls below 200 Plasmodium chabaudi infected mice develop a 60% higher parasitemia and liver injuries. The survival rate was much lower in mice with low taurine levels.
Potassium
Taurine also regulates the potassium levels in hepatocytes and erythrocytes. It reduces the release of potassium ions from cells.14,15 Potassium, the most abundant cation in the human body. On invading an uninfected human erythrocyte Plasmodium falciparum enters a low sodium, high potassium environment. It establishes new permeability pathways which allow the influx of sodium and efflux of potassium until reaching levels approaching those in the extraerythrocytic plasma.16 Erythrocytes in standard culture medium show a heavy invasion with young rings of previously uninfected red cells. In a medium of high potassium content this invasion was inhibited and many free merozoites were present.17 The voltage gated potassium channel plays an important role in parasite survival. Similar inhibition for sporozoites was also observed in high potassium medium. They lose motility and are inhibited in their progression through several hepatocytes.18 Among all the medicinal plants those of the Artemisia family have the highest potassium content. The first to report this was a USDA team.19 Potassium concentrations in mother’s milk are 2times higher at postpartum in colostrum than 1 month later in mature milk (Figure 1).20 In light of all this it becomes evident that there might be a link between the fat consumption belt and the malaria belt around the world.

Figure 1 In light of all this it becomes evident that there might be a link between the fat consumption belt and the malaria belt around the world.
Vitamin E
Vitamin E is a plant derived vitamin. It is possible that the high concentrations of vitamin E present in vegetable oils and not in animal fats plays an important role. Regulation of the vitamin E homeostasis occurs in the liver. The localization of tocopherol transfer protein TTP in the hepatocytes is dynamic and responds to the presence or absence of vitamin E. The function of hepatic TTP is to facilitate secretion of the ingested vitamin E from the hepatocytes, prior to its association with lipoproteins and delivery to non-hepatic tissues.21 Several studies have shown that the absence of vitamin E in a diet protects against sporozoite invasion and development. One of these studies has shown that mice fed E-containing diets during 4weeks prior the infection quickly died, whereas those fed the vitamin-E-deficient diets survived without developing detectable parasitemia. But already in 1957 similar results had been found. Cod-liver oils have a suppressive effect on infections of Plasmodium berghei in white mice and that the suppression is reversed by the oral administration of large amounts of vitamin E. The chicks were maintained on the experimental drugs for two weeks prior to infection.22‒24 Mice which lack the α-TTP liver cytosolic protein and which have very little vitamin E in circulation are highly resistant against malaria.25 The consumption of foods rich in vitamin E like peanut butter or moringa has probably to be reduced to avoid malaria infections. The same probably applies to vegetable oils rich in vitamin E or even vegetable gelatin capsules where at the Worcester Polytechnic Institute it was found that these food components significantly decrease the recovery of artemisinin, a strong oxidant, from the intestinal liquid phase, but not those of flavonoids.26,27 The effect may eventually explain by the well-known and strong reaction of vitamin E with peroxides.28 The intraerythrocytic stages of P. falciparum have an active pathway for biosynthesis of vitamin E. Based on these findings, a new drug target for antimalarial could be developed.29
Sulfur
It is well known since manyyears that the circumsporozoite protein (CSP) and the thrombospondin-related adhesive protein (TRAP) densely coat malaria (Plasmodia) sporozoites. CSP and TRAP are the antigenic targets of RTS, S, and a pre-erythrocytic malaria vaccine currently undergoing clinical trials. CSP contain an amino acid sequence that binds to heparan sulfate proteoglycans on the hepatocyte surface.
TRAP and CSP are also contributing to the gliding motility of sporozoites.30‒33 CSP is also able to bind with other sulfates like heparin or dextrose sulfate and these may inhibit sporozoite infectivity, motility and block an invasion of the hepatocytes. Sulfur containing molecules seem to play a key role. Hydrogen sulfide protects against cerebral malaria.34 H₂S has the reputation to be a toxic gas. But at low concentrations it has beneficial health effects and cures several diseases. Most of the balneary tradition is based on the presence of hydrogen sulfide in some mineral waters. The effect may be related to the precipitation of excess iron in the form of insoluble FeS.35,36 In mammalian cells, there is an endogenous production of H₂S. It is located in iron-sulfur clusters in the mitochondria and readily released when needed. In other words, hydrogen sulfide binds the excess of iron which is detrimental diseases, but renders it available when needed. Taurine generates hydrogen sulfide in the large intestine. A controlled feeding study on humans has confirmed this.37 Recently it has been demonstrated in vitro and in vivo that hydrogen sulfide and sulfur compounds can have an antiplasmodial effect of the same level as artesunate (Figure 2).38,39 A strong prophylactive effect was noticed for ABPS sulfated polysaccharides from Achyrantes bidentata where ABPS pretreatment clearly boosts response against the parasite (Figure 3) NC is the control, 0 d injection of ABPS on the day of infection, 10d and 15d injection 10 and 15days before inoculation by Plasmodium yoelii.40
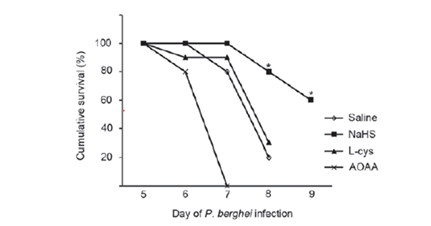
Figure 2 Cumulative survival of infected mice. Infected mice were treated with saline, 1mg/kg NaHS, 0.3mg/kg L-cys or 10mg/kg AOAA. L-cys, L-cysteine; AOAA, aminooxyacetic acid.
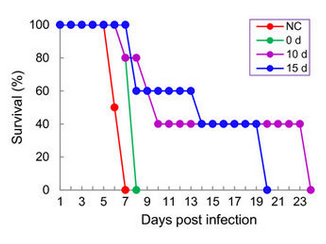
Figure 3 NC is the control, 0 d injection of ABPS on the day of infection, 10 d and 15 d injection 10 and 15 days before inoculation by Plasmodium yoelii.40
Taurine, and the two other sulfur amino acids, generate hydrogen sulfide in the large intestine. A controlled feeding study on humans has confirmed this.41 Sulfated polysaccharides also interfere with the invasion of another apicomplexan parasite like Cryptosporidium parvum in endothelian cells. Five sulfated polysaccharides were evaluated and heparin had the highest inhibitory effect. On the other hand, cells deficient in heparan sulfate show less susceptibility to invasion. It is as if you needed a sulfated key and a sulfated lock to open the door.42,43 Artemisia annua contains 0.4% of sulfur which is much higher than in most plants.44 A study from Senegal also finds that Artemisia annua plants are richer in sulfur than in phosphorus, iron, manganese, copper and zinc.45 Artemisia plants are also rich in polysaccharides: 2.15% in Artemisia argyi, 5.8% in Artemisia absinthium.46,47 As Artemisia plants are rich in sulfur and polysaccharides it is no surprise to detect sulfated polysaccharides in these plants. The polysaccharides of Artemisia tripartita contain 5% of sulfate.48,49 Sulfated polysaccharides not only inhibit the invasion of hepatocytes by sporozoites but also the invasion of erythrocytes by merozoites. Marine organism sulfated polysaccharides exhibit significant antimalarial activity. Optimal activity requires a degree of sulfation more than or equal to 2. Sulfated molecules like heparan sulfate occur naturally on the surface of human erythrocytes, where they may act as receptors for binding of merozoite surface proteins.50,51
Foods which are rich in sulfur: dairy products, meat, fish. Most vegetables are very poor except a few like garlic, avocado and cacao. Essential oils and aqueous extracts of garlic are very high in sulfur compounds, up to 90% for the oil. If sulfur plays a role in prophylaxis these garlic extracts must be active.52 The ability of allicin to inhibit sporozoite invasion and malaria infection was investigated in Israel. When sporozoites were treated with allicin before inoculation into mice, malaria infection was completely prevented. Mice injected with allicin had decreased Plasmodium infections compared to controls.53 In allicin treated mice the number of CD4+ T cells and macrophages is much higher. This is in line with our findings in Katanga where we confirmed that administration of capsules containing Artemisia leaf powder raised the CD4⁺ and that this had a significant antimalarial effect.54 The aqueous extract of garlic (Allium sativum) is also a strong beta-hematin inhibitor (IC50 0.2mg/L). Garlic does thus have, not only prophylactic, but also curative properties, or in other words garlic and allicin can target two different life cycle stages in the vertebrate host. This has been documented in other papers.55,56 The antimalarial activity of garlic oil has been recognized by the pharmaceutical industry and assays have been run in mice to study the combined effect of garlic oil and arteether. Mice treated with single dose intramuscular injection of arteether at 750µg in combination with three 100µL oral doses of garlic oil on day 3, 5 and 7 gave 100% protection of survival.57 Cocoa (Theobroma cacao) was known by the Maya as diet-mediated antimalarial prophylaxis. Based on this anecdotal information prophylactic trials have been started in Ghana by the Ghana Cocoa Board. People are encouraged to daily drink a beverage made by mixing boiling-hot water and natural cocoa powder.58 Natural cocoa powder has measurable direct in vitro inhibitory effect on Plasmodium falciparum. This supports anecdotal reports of its ability to prevent malaria, as a result of regular beverage intake.59 This has been confirmed by in vivo trials in mice. Cocoa powder was equivalent to chloroquine and has also prophylactic properties.60,61
Sulfur is the sixth most abundant macro mineral in breast milk. Cow’s milk may contain 20µg/kg in the form of hydrogen sulfide and methanethiol. Sulfur content is probably higher in human milk where the concentration of the sulfated amino acid taurine is 6times higher than in cow’s milk. The fact that breastfed infants stay free of malaria during 6months is an argument supporting the role of the high taurine content in human milk. Breast milk is a remarkably “altruistic” secretion, that is, its contents are directed at protecting the infant with minimal benefit to the mother. The concentration of antibodies, mainly IgA, is 10-100 folds higher than in serum. In colostrum it is as high as 90g/L. This is important as infant intestinal IgA production does not begin until several months of age.62,63
Thousands of research papers have been written on artemisinin. But this molecule has no effect on sporozoites and gametocytes, on prevention and transmission. Curative and preventive strategies for malaria treatment should ideally target three malarial life-cycle stages: exo erythrocytic forms, the asexual blood stages, and the transmission stages.
None.
Author declares that there is no conflict of interest.

©2017 Lutgen. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.