eISSN: 2379-6367


Review Article Volume 12 Issue 4
1Department of Materials Engineering, Indian Institute of Science, Bangalore, India
2Department of Mechanical Engineering, Faculty of Engineering, National University of Laos, Sokpaluang 01005,Vientiane, Laos
Correspondence: Nipun Jain, Department of Materials Engineering, Indian Institute of Science, Bangalore, Karnataka, 560012, India, Tel +91 9540209811
Received: August 06, 2024 | Published: September 3, 2024
Citation: Jain N, Waidi YO, Debnath S, et al. Mechanotransduction alterations in tissue-engineered tumor models for new drug interventions. Pharm Pharmacol Int J. 2024;12(4):148-165. DOI: 10.15406/ppij.2024.12.00446
Mechanotransduction is a collection of pathways in which the cells reprogram themselves by sensing mechanical stimuli. Cells use biological cues to interpret the physiological stresses and respond to changing conditions by modifying the cellular and ECM architecture. This feedback loop regulates a variety of cellular processes, including migration, growth, differentiation, and death, which is essential for the network stability to work together in a coordinated manner. The effect of stress on cancer progression and the role of mechanics as a critical inducer in determining the cancer cell fate has been studied. This review discusses the progression of cancer cells to epithelial to mesenchymal transitions. It examines tumor microenvironment models, such as spheroids, bio-printing, and microfluidics, and how they recapitulate the tumor microenvironment. These offer certain benefits and help replicate the fundamental behavior in vivo conditions. We further discuss mechanosensing, the associated signaling molecules, and how it modulates the cancer drug resistance and transduction pathways that implicate cancer treatment. The difficulties with the existing methods and the prospects for additional study that may be applied in this area are discussed, and how they allow for new therapeutic development.
Graphical abstract
Keywords: mechanotransduction, mechanotherapy, tumor dynamics, cancer-on-a-chip, 3d-bioprinting, disease model
In the 21st century, there has been a greater emphasis on understanding the influence of mechanical forces in signal transmission and gene expression to understand cellular reactions in physiological conditions,1,2 specifically in developing new cancer treatments. The term "mechanotransduction" refers to the physiological mechanisms that allow cells to respond to their external environment by converting mechanical stimuli into biochemical signals. Mechanotherapy hinges on mechanotransduction, where mechanical forces applied to cells trigger a coordinated response involving multiple cell groups.3 This process starts with the local transfer of the mechanical signal (mechano-transmission), which rapidly spreads through the interconnected network of proteins within the cell, typically within milliseconds.4 Under prolonged or strong stressed conditions, these forces can even deform cellular structures, prompting them to adapt and strengthen themselves. These structural changes trigger a cascade of biochemical signaling events (mechano-sensing), ultimately determining the cell's fate.5 The cytoskeleton and cell membrane are vital in transmitting these signals, ensuring a controlled mechanoresponse that dictates whether the cell survives, adapts, or dies.6
Unlike other signaling systems that operate on rapid timescales, mechanoresponsive pathways are relatively slow, with transmission routes taking minutes and gene expression changes lasting hours to days.7 Key players in this process include cell surface receptor proteins, cytoskeletal components, and extracellular matrix proteins. Multiple theories have been suggested that reveal how external forces trigger mechanotransduction, affecting tumor behavior by boosting cell death, slowing growth, and inhibiting movement. These forces, along with varied physical conditions such as hypoxia and angiogenesis, are essential for the growth of tumors. 2,8 The complexity of applied "pressure" regarding tumors is evident in diverse contexts.
The stresses, such as external forces, fluid pressure within tissues, and dynamic interactions between cell populations, are crucial for tissue stability. As tumor volume grows, an intrinsic interplay between cells, their surrounding matrix, and internal components leads to elastic adjustments. External factors also play a role by applying static or dynamic compressive pressures that can hinder tumor growth and even induce cell death.9 All these investigations concluded that compressive stress effectively reduces tumor growth. Compression between 5-10 kPa significantly reduced tumor cell proliferation (by at least 50%) and boosted apoptosis (30%) compared to stress-free growth. This suggests that the surrounding environment can pressure tumors, and external or internal stress can significantly alter their behavior. High-pressure areas hamper proliferation, leading to unique growth patterns. Additionally, peripheral cells in a monolayer could transform into "leader cells" that initiate collective migration even though mild ultrasound-induced compression seems to hinder migration.10 One way to study tumors is by mimicking their environment in the lab. Researchers can grow tumor cells in engineered 3D tumor models. This method reveals the importance of stiffness within a tumor and how it affects its growth and surrounding matrix deposition.11 Internal forces arise from growth, reorganization, and adaptations between the cells and the matrix. These forces can influence tumor patterns, metabolism, and drug delivery.12 The stress distribution within a tumor is complex. The core experiences radial and circumferential compression, while the tumor and surrounding tissue interface experiences compressive and tensile stresses in different directions.13
Certain review papers are present that explain the importance of ECM,14 stiffness,15 and signal transduction pathways.16 We describe the fundamental ideas guiding the cancer stress response and concentrate on the main theories underlying "mechanotherapy," a promising strategy that uses mechanical forces for therapeutic advantage, even though the precise mechanisms differ depending on the kind of cell. This review further explores the principles of mechanotransduction, focusing on 1. The biomechanical aspects of the tumor microenvironment; 2. Tissue-engineered tumor models; 3. Key signaling pathways involved in mechanotransduction; 4. The impact of mechanosensing on cancer drug resistance and its therapeutic implications. By comprehending these mechanisms, scientists might create novel treatment approaches that concentrate on mechanical elements, providing encouraging prospects for battling cancer and enhancing patient results.
Role of changing ECM and microenvironment in governing cancer progression
The extracellular matrix is a dynamic web of proteins that is a dynamic entity that is constantly undergoing remodeling.17 This dynamic process relies on the coordinated actions of matrix metalloproteinases (MMPs), adamalysins, and meprins, which cleave ECM components and dictate their abundance and organization.18 This intricate mechanism ensures the ECM's adaptability and responsiveness to various physiological cues, impacting cell behavior and overall tissue function. This intricate breakdown can have profound consequences for cancer. By releasing trapped growth factors and cytokines, ECM degradation can fuel tumor growth, blood vessel formation (angiogenesis), and inflammation. The number of ECM elements also matters, directly impacting tissue density and stiffness.19
Effect of changing ECM stiffness by collagen and elastin
Collagen and elastin, two essential building blocks of the extracellular matrix (ECM), are crucial in determining tissue stiffness. Studies have shown that activating TGF-β, IGF/IGF-1R, and PI3K/Akt signaling pathways enhances ECM protein production, ultimately increasing ECM proteins like collagens.20,21 Inside cells, a molecular chaperone called Hsp47 assists in properly folding and processing procollagen and boosts collagen secretion into the ECM.22 Another key player is SPARC (Secreted Protein Acidic and Rich in Cysteine), a chaperone that works directly within the ECM. SPARC binds to collagens, protecting them from degradation and ensuring their proper formation.23 Therefore, these intracellular and extracellular chaperones are critical in ensuring proper secretion and positioning of ECM proteins. Beyond simply producing ECM components, precise changes at specific locations within collagen molecules are crucial for their solubility and alignment within the matrix. The density and arrangement of these fibers, along with those of elastin, ultimately determine the overall stiffness of the ECM. The LOX enzyme further fine-tuns this stiffness, which modifies collagen and elastin precursors through oxidative deamination. This modification results in the formation of allysine residues, which contribute to the strength and stability of the ECM.24
Interestingly, a growth factor called TGF- β1, notorious for its pro-fibrotic effects can ramp up the production of LOX that catalyzes this cross-linking, particularly in cancer tissues.25 Tissue transglutaminases also orchestrate collagen cross-linking and work with LOX to amplify tissue stiffness.26 Furthermore, increased collagen deposition by cancer cells and fibroblasts potentially drives prolyl 4-hydroxylase alpha-1/2 (P4HA1/2) expression and increases rigidity by aligning the deposited collagen fibers.27 In organs like the pancreas and liver, specialized stellate cells contribute to stiffening by amplifying LH2 expression.28 While some matrix proteins like fibronectin act as brakes, inhibiting the activation of key players like hepatic stellate cells in response to TGF can actively drive the stiffening of the ECM.29 ROCK acts as a mechanosensor, translating collagen, fibronectin, and periostin deposition via beta-catenin signaling.30 Even aged mesenchymal stem cells (MSCs) can add to the stiffness chorus, contributing to a dense collagenous melody in the tumor microenvironment.31 However, the influence of matrix stiffness is a two-way street. This rigidity dictates the fate of mesenchymal stromal cells and also tweaks their tune, turning them into active supporters of the tumor's growth. Growth factors like PDGF amplify the mammary tissue's stiffness, boosting hyaluronic acid and collagen production.32 This intricate interplay between matrix rigidity and growth factor production plays a pivotal role in cancer progression, influencing its growth, immune evasion, and resistance to treatment.
The matrix dynamics in the TME
The environment surrounding cancer can promote or restrict cellular activities such as migration, polarisation, cytoskeletal organization, and growth through mechanical signaling. Tumor microenvironment cells control extracellular matrix remodelling and improve the associated characteristics during the invasion. Often found in solid tumors, cancer-associated fibroblasts are a dynamic subset that drives the growth of tumors.33 CAFs stimulate invasion proportionately, depositing fibronectin, necessitating matching αvβ3 integrin expression. Additionally, it appears that CAFs activate the mechanistic regulator of the oncogenic cells, which in turn increases ECM stiffening and controls the actomyosin cytoskeleton.34 Thus, a progressive loop involving CAFs with the matrix is maintained to control tension forces. CAFs use a heterophilic junction between E-cadherin (cancer-specific) and N-cadherin (CAF-specific) to transfer physical force, which encourages cellular invasion.35 Additionally, CAFs apply mechanical stresses and extend the pre-existing breaches to facilitate the invasion of nearby tissues.36 LH2, a protein released by cancer-associated fibroblasts (CAFs), is a key factor that stiffens the stroma. This increased rigidity, driven by collagen cross-links, facilitates cell invasion and metastasis.37 LH2 overexpression is a common thread in various cancers. Several transcription factors, including SMADs, GATA3, and HIF-1a, directly boost LH2 production.38
Beyond genetic factors, the physical environment surrounding cancer, known as the tumor microenvironment (TME), is important in cancer development. This environment, characterized by factors like reduced oxygen levels (hypoxia), can intricately influence the stiffness of the surrounding tissue matrix.39 Notably, even systemic health factors like obesity can indirectly affect breast tissue stiffness through changes in the adipose tissue microenvironment. This stiffened matrix acts as a communication hub, sending mechanical signals to various cell types within the tumor and its vicinity. These signals can trigger a cascade of processes, including the transformation of normal cells into cancer, the breakdown of cellular components for energy (autophagy), EMT induction, increased cell invasion, and altered metabolism.40 The emerging field of pharmacological intervention and therapeutic strategies in matrix stiffness offers a potential approach to restricting cancer before it fully develops.
Molecular changes in the ECM during metastasis
One distinctive characteristic of cancer cells is their capability to travel from their original site to neighboring or faraway locations.41 This process usually entails a sequence of steps starting from EMT (Figure 1). As the cancer cells transform, they start to infiltrate neighboring sites, may even enter the blood for circulation (intravasation), or escape the circulatory system (extravasation) to start and finish the metastatic cycle.42 Stephen Paget's "seed and soil" theory is one of the most well-known theories. It implies that the interaction between the disease and the particular organ microenvironment drives the spread of cancer. This widely established theory serves as a roadmap for studying cancer and metastasis.43 The critical function of the mechanotransduction feedback loop is to mediate the interaction between biophysical stimuli and biochemical responses.
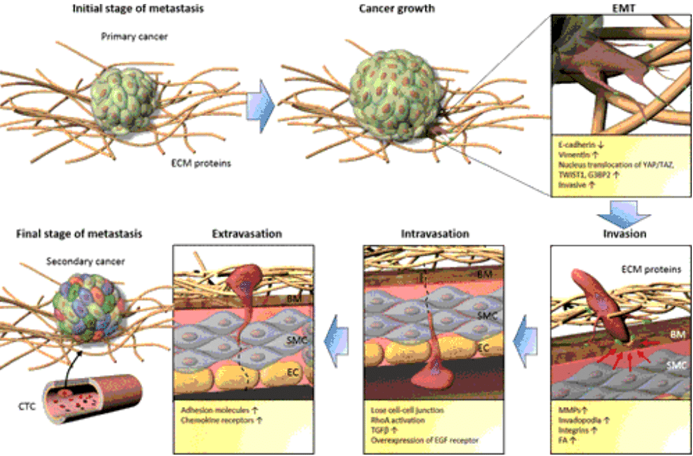
Figure 1 A diagrammatic illustration of cancer metastasis. Reproduced with permission from.42 Copyright 2019, American Chemical Society.
Alterations in matrix stiffness can modulate the physical properties. This shift is not passive but mediated by "mechano-transducers" that pick up on these mechanical signals. These sensors, often ion channels like TRPs and Piezos, act like translators, turning the physical pressure of a stiffer ECM into chemical signals. This primarily happens through calcium signaling pathways, impacting tumor cells and the surrounding tissue (stroma).44 One key player in this translation process is integrin, a protein that acts as a mechanical bridge between the cell and ECM. When Piezo channels sense stiffness, they activate integrins, triggering a cascade of events within the cell. Scaffolding proteins like vinculin, talin, and paxillin link with signaling molecules like FAK, Src, and PI3K/Akt. This orchestrated response controls how the cell builds focal adhesions, anchoring points to the ECM and rearranging its internal cytoskeleton.45
Furthermore, this stiffening can activate a protein called Rho-associated protein kinase (ROCK) that enhances signaling pathways like integrin and MAPK. These pathways ultimately lead to increased protein stability called SNAIL.46 Interestingly, SNAIL and other related proteins contribute to the overexpression of another key player, YAP. This remains a crucial mechano-transducer that translates mechanical cues into biochemical signals. Its activation can even boost the expression of a mechano-sensor Piezo1, thereby creating a feedback loop.47 It's important to note that YAP sometimes responds to a stiff ECM, highlighting the complexity of these pathways. However, when it does, the resulting signaling diversity in both tumor and stromal cells can fuel several hallmarks of cancer progression, including tumor development, angiogenesis (new blood vessel formation), metastasis, immune evasion, and even resistance to treatment.48 YAP activation, downstream of integrin and Piezo1, can enhance cell migration by promoting a specific pathway and the production of MMP-7.49 TRPV4, another mechanosensitive channel, is a potential driver of cancer's intricate ability to sense the rigidity of the environment and remains a critical phase in metastasis through interactions with other key proteins.50
Additionally, the ephrin receptor EPHA2, regardless of its binding partners, can activate a signaling cascade leading to Twist1 phosphorylation and nuclear translocation, ultimately driving EMT and metastasis.51 While a stiff ECM generally promotes cancer progression, it's important to note that the story isn't always so straightforward. Some studies suggest that a very soft ECM can also facilitate invasion. This can happen through mechanisms like reduced cell adhesion and increased production matrix metalloproteinases (MMPs) that break down the surrounding tissue, thus clearing the path for cancer cells to spread.52 Interestingly, a loss of a specific protein called vacuolar ATPase 'a2' in mammary tissue can lead to softer ECM and, surprisingly, induce metastasis.53 A deprived ECM glycosylation often leads to decreased stiffness, causing a rise in stiffness-independent factors and pro-metastatic effect. Unraveling the intricate puzzle of cancer cell adaptation to ECM rigidity changes requires deeper research. Further research into the mechanotransduction mechanisms and their role in cancer can lead to novel therapeutic approaches, improving patient outcomes.
The use of tissue-engineered models in studying cancer
The focus in recent years has shifted from decades of research to understanding the relationship between the microenvironment of tumors and malignancy. The TME matrix comprises several macromolecules and interwoven cell-scale fibrils.54 The cell core stress contributes significantly through their cooperative relationships with other constituents in addition to providing physical support, rigidity, and topographical layout (Figure 2). The provenance and reaction to mechanical stimuli vary in intensity, direction, and duration among different cell types. However, there needs to be more knowledge of the mechanics and applications of mechanotransduction. Recent research has concentrated on lowering disease by concentrating on detrimental mechanical components in the TME.55 TME research mostly focused on biochemical signals, including low pH, inflammation, hypoxia, and immunosuppression, ignoring mechanical cues (Table 1). The tumor's "lifetime" is impacted by the differences between the TME and the normal tissue microenvironment. Mechanical factors have a major impact on the development of tumors and dissemination. Tissue engineering strategies provide a wide range of tools for replicating the mechanical forces studied in cancer. These 3D cancer models can be employed for preclinical drug screening and cancer pathway investigations.56 In this topic, we briefly examine the choice of selection in materials for constructing tumor models, followed by an exploration of recent advancements in tissue engineering aimed at replicating the biomechanical forces.
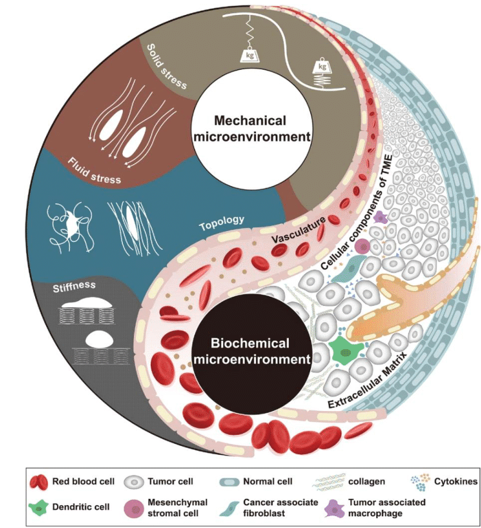
Figure 2 The biochemical and biomechanical properties of the microenvironment control the fate of cancer cells. Reproduced with permission from.58 Copyright 2022, John Wiley and Sons
|
Signaling cue |
Mechanism |
Protein mediator |
Significance |
|
Biochemical |
|||
|
Hypoxia |
There needs to be more oxygen usage and more supply. |
HIF-1α |
Cellular death Drug-resistance |
|
Acidic pH |
Mostly, anaerobic metabolism causes high-pressure interstitial fluid, low glucose, hypoxia, and high lactate. |
Glycolytic enzymes PDK1 PKM2 |
Improved tumor invasion. DNA instability. Radioactivity, medication resistance. Immune escape. |
|
Inflammation |
The production of inflammatory mediators |
NF-κB STAT3 HIF-1α |
Tumor angiogenic. Increased tumor growth. Cancer apoptosis. |
|
Mechanical TME |
|||
|
Solid stress |
This can cause cell proliferation, matrix deposition, and contraction. |
Collagen MMPs Actomyosin |
Challenging drug delivery Higher tumor cell migration Tumor angiogenesis Stopping mitosis. |
|
Fluid stress |
Cell proliferation, contraction, and matrix deposition. |
VEGF Glycocalyx E-cadherin |
Angiogenesis Higher invasion Complicated drug delivery |
|
Stiffness |
Matrix deposition & cross-linking |
Collagen MMPs |
Metastasis, invasion. Improved infiltration of immune cells |
|
Topology |
Cell contraction, matrix deposition, and cross-linking |
Collagen MMPs Actomyosin |
Metastasis, invasion. |
Table 1 Biochemical and biomechanical cues in the tumor microenvironment
Choice of material in 3D Tumor Models:
Scaffold materials can modulate the transcription level of certain genes in the 3D cancer model. Several studies have focused on analyzing the type of materials, rigidity, shear stress, and dynamic mechanical stretch to study the cell and tissue response to cancer development and drug therapy resistance.57 Mechanical (substrate elasticity and rigidity), architecture (surface topography), chemical (ligand, bioactive signals, and degradation), and ECM composition affect the cell self-organization, proliferation, and migration in their 3D matrix. Natural ECM components are preferred over synthetic material components for better resemblance of tissue architecture. Matrigel (Engelbreth-Holm-Swarm sarcoma-derived components) is frequently used to prepare 3D culture systems. However, these are only feasible sometimes due to their cost and fluctuating composition across batches.58 Thus, synthetic materials become increasingly desirable due to their affordability, production flexibility, control over rigidity, degradability, and sticky ligands. Mechanically tunable hydrogel models allow intestinal stem cells to multiply and differentiate into organoids. Mechanical stresses are highlighted in Table 2, in which 3D cancer models that assess tumor microenvironment are described.
|
Model |
Mechanical environment |
Outcomes |
|
Ovarian cancer gelatin-methacrylamide spheroids |
0.7-16.5 kPa substrate stiffness. |
• The ECM mechanical environment controls cell proliferation and migration. Paclitaxel alone and with ATN-161 affect implantable spherical hydrogels. |
|
HCC collagen-coated |
1–10 and 6–16 kPa substrate stiffnesses |
Stiffness increases • Increases VEGF expression and promotes angiogenesis. • Expression of osteopontin rises. • Activates the TGF-β1 • Increases stemness-related genes. |
|
Electrospun PCL ES |
Shear stress from flow: 1.7–17.0 cPa |
Enhances IGF-1 and lowers HER2 expression, increasing dalotuzumab resistance. |
|
Hyaluronic acid and porous collagen ES |
(1% or 10% strain) unconfined dynamic compression |
Stimulates ERK1/2-dependent RUNX2 demonstration, Sorafenib resistance rises. |
|
A decellularised jejunum culture of colorectal cancer and fibroblasts |
Flow-persuaded shear stress: 3.0–5.0 mPa |
Decreased vimentin and increased E-cadherin expression, stimulating EMT |
|
In porous mineralized poly (lactic-co-glycolic acid), breast cancer-conditioned MSCs |
Dynamic 10% peak strain compression |
• Compressive filling increases osteopontin synthesis by signaling tumor-derived factors via paracrine pathways. • Promotes metastasis through tumor-stroma interactions. |
Models depicting cellular interaction with ECM stiffness
One intrinsic characteristic of the microenvironment that needs to be recapitulated is the stiffness. Research showed that it may vary greatly among tissues, ranging from 1 - 70 kPa.59 ECM variability is also observed at various growth levels due to ECM protein deposition and breakdown differences. The stiffness of ECM is determined by the volume and quality of cross-linking in fibers. Tumor cells are the mechanical cue providers, and distinct structural components generate solid stresses along the microenvironment.12 The microscopic exchanges between the structure's elements create cell proliferation-induced tension (residual stress), and the tumor tissue continues to experience it even when the tumor is eliminated.60 Research has demonstrated that solid tension within the TME can develop negative impacts such as blood artery compression, increased fluid pressure, and ECM remodeling.61
Solid stress accumulation has numerous implications in clinical disease as the generated tension causes neurological damage, invasion, and movement.62 Though the precise mechanisms behind the association of tissue rigidity with carcinogenesis remain unresolved, an increase in rigidity constitutes one of the most significant clinical indicators. In vitro, simulations of the interplay between matrix tension and cells are widely recognized to understand the effect of ECM rigidity. In one case, as a bionic 3-dimensional Matrigel culturing system, the modulus of elasticity varied from about 150 Pa to 5700 Pa in cancerous tissues. Twist1 transcription factor was discovered to be increased by increasing ECM matrix rigidity, which directly enhanced EMT, invasion, and metastasis.63 The in vitro specimen with adjustable collagen rigidity was constructed considering that the density and level of interconnection in collagen are major parameters determining the rigidity of the ECM. Researchers have determined that enhanced matrix rigidity with cross-linked collagen affects angiogenesis and vascular development.64
The changing stiffness also governs the formation of new blood vessels and could further deteriorate the effectiveness and shipment of therapeutic therapies. Some innovative technologies have been employed to explore the function of mechanical indicators. The in vitro interactions between cells and substrates are characterized by force exertion, detection, traction force microscopy (TFM), and micropillars for learning more about the forces of mechanics.65 Kourouklis et al. used TFM with cell microarrays onto a range of stiffness substrates for assessing cell-generated traction forces and their phenotypic response.66 Researchers have also found that rigidity characteristics and matrix protein levels regulated cholangiocyte distinction. They also discovered how ERK and Rho-associated protein kinase (ROCK) function in this process. Tian et al. employed PA substrates to mimic the matrix stiffness of different tissues, ranging from soft brain to hard bone. They also examined the kinetic responses of cancerous breast cells with magnet tweezers and advanced imaging methods to different stiffnesses.67 Micropillar substrate production provided a solution to the fundamental drawbacks of a substrate with constant elasticity. This approach has been widely used to investigate the linking cell reactions and matrices rigidity to measure the cell-generated forces.68 Microforce sensing micropillars, for example, detect deflections of tens of nanometers and transform measurements into real force values.68
Models depicting cellular interaction with changing stress during blood flow
Numerous studies have examined the role of biochemical indicators in tumor vasculature networks and have shown how fluid stress results from abnormalities. Fluid stress, usually given by blood and interstitial flow, comprises three components: shear stress, microvascular fluid pressure, and interstitial fluid pressure.12,58 Due to the leaky and distorted nature of the tumor vasculature, there is insufficient perfusion, an inadequate supply of oxygen or nutrients, and an increased geometric and viscosity resistance to blood flow.
Effects of changing shear stress
The stress of the interstitial liquid varies from nearly 0 in most regular tissues to around 60 mm Hg in areas with neoplasms and up to 130 mmHg in rat pancreatic ductal adenocarcinomas.70 The interstitial liquid pressure is higher within the central tumor areas; it moves beyond the core to its outskirts and transports proangiogenic chemicals, like the proliferation of the vascular endothelium. Such proangiogenic medications further raise the possibility of lymph node metastasis.71 The stress of the interstitial blood governs the prognosis in certain melanoma, lung cancer, lymphoma, and cervical cancer. The circulation exerts a tangential force (shear stress) on the vascular endothelial lining. Shear rate and blood viscosity both affect the endothelial cells.72 When tumor cells travel to distant tissues, their primary obstacle is shear stress. Through a number of important mechanotransduction pathways, shear stress promotes tumor cell adhesion, motility, and invasion during metastasis.73 It has been demonstrated that absolute tension stimulates the signaling pathways for ERK1/2 and FAK to encourage stem cell motility from liver cancer.74 Furthermore, it has been documented to trigger autophagy, promoting HepG2 cell motility and invasion via the integrin/cytoskeleton route.73 Moreover, it was shown that lymphatic shear stress overexpresses Yes-associated protein, which encourages cancer cell migration.75
The migrated cells become floating cells in the bloodstream veins as they reach the vascular system. They encounter high fluid shear stress and are easily influenced by blood circulation.76 Therefore, it is essential to comprehend how tumor cells survive in circulation to prevent tumorigenesis and metastasis. Numerous models have been developed in order to achieve this. In an in vitro circulation system, a silicone microtube, syringe, and peristaltic pump usually create pulsing flow, mimicking fluid shear stress in vivo. This is equivalent to the shear stresses in the venous and artery circulations. The majority of the migrated cells may be eliminated by shear stress in blood circulation in less than 12 hours.77 The fluid stress also promotes EMT, which raises residual suspended survival through B cell lymphoma and Puma.78 According to research, important laminin components are overexpressed and survive by reducing fluid shear stress.79
Effects of changing microvascular pressure
Native tumor tissues have a variety of ductal structures produced by extracellular matrix remodelling that act as barriers to cell invasion and migration during tumor metastasis. Various methods, such as grooved substrates, parallel plates, and microchannels, have investigated how ECM confinement affects tumor cell migration.80 A few years ago, there was a lot of interest in a microculture substrate to investigate the impacts of rigidity and restriction on the motility of tumor cells. Using photolithographic methods and adjustable formulations of polyacrylamide-based hydrogel, a group of individuals created micro-PA channels alongside predetermined rigidity. These micro-channel diameters varied from 10 to 40 μm and 0.4 to 120 kPa in stiffness. Employing atomic force microscopy (AFM), they found that higher traction polarisation of cells causes them to migrate more quickly in smaller than broader channels and eliminates the need for surface rigidity.81
Patteson et al. created microfluidic devices to simulate 3D tissue cell motion. These pores are adequate to maintain the vimentin network and are also large enough to allow cells to flow past. The absence of vimentin lowers cell rigidity and also encourages the movement of 3D cells with tiny confining areas, based on their examination of the cellular basement reticulum's and the perineural area's rigidity to examine the mechanical response of the cells.82 Naturally occurring tissue includes small channels with a diameter of less than 10 μm, for example, perineural tissues, beyond the ductal structures produced by extracellular matrix remodelling. During tumor metastasis, these microchannels act as spatial confinement for cell motility.83 The small channel platforms based on hydrogel that have adjustable channel width (3-11 μm) and changeable ECM rigidity (0.3 to 20 kPa) were created by the integration of photolithography technologies using an alginate-collagen copolymer in order to look into cancer movement. Using this platform, researchers showed that the width and rigidity of the channels combine to limit the migratory speed and the shift in movement modes among mesenchymal cells.80
Methods that simulate restrictions make it possible to investigate tumor cell movement and its relationship with the surrounding environment using physical cues. The nucleus remains the primary barrier for cellular movement across small spaces, as it is stiffer than other cell parts. A microfluidic setup was commenced to explore the connection between channel dimensions and nuclear passability. According to this work, cells have a threshold width below which they cannot pass through tiny gaps, and their capacity to do so decreases as microchannel confinement increases.84 With the ability to image the nucleus and the shape of the cell in real-time during translocation, this microfluidic device helps to study cell adaptability in a constrained environment.
3D tissue model fabrication techniques
Traditionally, the quest for new cancer treatments relied heavily on two approaches: flat, two-dimensional (2D) cell cultures in the lab and miniature animal models85 However, capturing the complex reality of tumors within these simplified settings proved challenging. 2D cultures struggled to replicate the intricate tumor microenvironment, while concerns about animal models' cost and ethical limitations dampened their appeal for routine drug testing.86 The advancements in biotechnology have ushered in a new era in cancer research by promoting three-dimensional (3D) in vitro models.87 These sophisticated models offer a more faithful representation of the natural tumor microenvironment, more accurately mimicking its intricate architecture and cellular interactions. This explains why most drugs effective in animals fail to work in clinical trials.88 These findings highlight the importance of 3D models that mimic the human body and allow real-time drug response observation. Alternatively, cells cultured in a 3D system can interact and behave more similarly in the body, including adhesion, motility, invasiveness, and metastasis.89 The following section delves into various tools and materials currently employed to construct 3D cancer models. Numerous platforms have been established to unravel these transduction pathways. Each method's unique advantages and limitations are thoroughly examined.
Figure 3is a visual guide illustrating the various processes and components of crafting these intricate models. Researchers have developed diverse approaches to address various challenges, including cancer-on-a-chip platforms, 3D bioprinting, and spheroid cultures.90
Spheroids and hanging drops
Culturing tumor cells in 3D environments allows the formation of multicellular tumor spheroids (MCTS) that stand out for their simplicity and effectiveness.91 These spheroids, which resemble miniature tumors, offer valuable insights into drug response compared to traditional 2D cultures. Several techniques exist for generating MCTS, each with its advantages and limitations. Popular methods include hanging drop, spinner flask, micropatterned plates, and magnetic levitation.92 Choosing the optimal method depends on factors like production efficiency, size uniformity, impact on cell behavior, and compatibility with downstream applications.93 The hanging-drop method is particularly attractive due to its remarkable simplicity, as it allows for natural cell aggregation.94 This method utilizes gravity within tiny pipetted droplets to concentrate cells at the liquid-air interface. While the hanging-drop technique boasts ease and affordability, challenges arise in scaling up production and maintaining consistent droplet size and uniformity. Microfluidic chips have emerged as a promising alternative, but maintaining long-term cultures on these spheroid-on- chip devices remain challenging.95 While the hanging drop approach is straightforward and uses manual pipetting, its significant time and labor requirements might pose limitations.96
Furthermore, these technical limitations exert mechanical stress, affecting the droplet shape and culture structure. A study offered a novel and highly effective method for generating cell culture spheroids.97 This technique exploits the differential physical properties, namely surface tension and density, of two immiscible liquid solutions. Researchers can sculpt and precisely control spherical indentations within a pliable elastomeric PDMS substrate by manipulating these properties. Moving beyond traditional flat cell cultures, researchers are turning to 3D tumor spheroids to replicate the TME. In this innovative study, scientists developed a method for co-culturing fibroblasts (NIH/3T3) and human kidney cancer cells (A498) in varied ratios within microfluidic wells.97 This approach yielded an impressive 97% success rate in generating individual tumor spheroids per well. It established the platform’s effectiveness for drug testing and monitoring reactive oxygen species. This breakthrough demonstrates the potential of this technology for studying tumor cell responses to drugs, paving the way for simpler and more relevant cancer drug screening.
3D Bio-printing
In the past few years, cell preservation, stem cells, and cancer research have experienced a huge interest in exciting bioprinting advancements. The ability to meticulously arrange and architect complex features within the tumor microenvironment allows researchers to control the spatial arrangement of 3D cancer cells.98 This technology holds the potential to not only recreate the 3D TME with intricate cell arrangements but also extend to tissues and organs, paving the way for a future of personalized medicine. The materials used in this process, called bioinks, are typically polymer hydrogels containing living cells or biomaterials. Three main approaches that have emerged for creating desired 3D structures are discussed below.
Extrusion-based bioprinting (EBB)
The advances in bioprinting have emerged with the development of EBB. This technology utilizes a fluid distribution arrangement coupled with a robotic mechanism for extrusion, offering researchers a versatile tool for creating complex 3D tissue models (Figure 4). EBB relies on three established methods: pneumatic, piezoelectric, and screw-driven mechanisms. It involves a controlled distribution system that meticulously delivers bio-ink-containing living cells to form cylindrical filaments. These filaments are then strategically deposited to generate intricate 3D structures, replicating the desired tissue architecture.99 This method offers high biocompatibility, dramatically reducing cell injury. Grolman et al.,99 effectively harnessed the power of EBB to establish a co-extrusion bioprinting setup. This technique allowed them to investigate the intricate interplay between tumor microenvironment macrophages and MDA-MB-231 breast cancer cells, offering valuable insights into cancer progression and potential therapeutic targets.100
Droplet-based bioprinting (DBB)
This technique has risen for engineering complex tissues, particularly for studying tumor microenvironments. This method leverages acoustic droplet ejection and microvalve technology to precisely control cell dispersion and density within printed structures.101 DBB builds upon established bioprinting principles, employing various droplet ejection mechanisms like thermal, piezoelectric, acoustic waves, or solenoid pumps to achieve similar outcomes (Figure 4). Researchers have successfully utilized DBB to create intricate tumor microenvironments. One study that employed this technique consisted of a central tumor spheroid enclosed by CAFs, allowing the investigation of interactions between the tumor and the surrounding stroma during invasion.102 Furthermore, DBB has been adapted to design microfluidic systems for the high-throughput production of uniform tumor cell spheroids, facilitating large-scale studies of tumor biology.102
Laser-based bioprinting (LBB)
LBB offers a cutting-edge alternative to previous techniques for crafting intricate 3D objects and directly writing on tissues. LBB uses a pulsed laser to deposit less viscous inks, enabling superior precision and control over pattern formation (Figure 4). This opens up exciting possibilities, as Kingsley et al. demonstrated by precisely tailoring the size of tumor spheroids.103 Further pushing the boundaries, a research group employed a high-throughput LBB to develop a tumor model.104 Co-culturing ovarian cancer cells and fibroblasts paved the way for more sophisticated and realistic models of complex biological systems. This method permitted the continuous modification of the spatial separation of multiple cell types. Traditional 3D bioprinting methods, such as inkjet and laser printing, offer some control over cell placement but pose limitations for precise, dynamic manipulation.105 While they’ve shown promise in distributing cells within 2D and 3D models, these techniques often require specific design parameters to avoid harming heat-sensitive cells and biofluids. This becomes especially crucial when printing live cells vulnerable to high temperatures and stress.106 Additionally, achieving directional control with standard inkjet devices can be challenging due to nozzle geometry and ejection limitations.
Cell assembly with hydrogels, matrigel, and magnetic nanoparticles
Hydrogels are a cross-linking polymer network with an extremely porous structure that absorbs up to 99% water. They closely resemble the physical characteristics of natural ECM and have extensive uses in cell cultivation techniques.107 The hydrogel preparation involves the use of several natural and synthetic polymers. Collagens, hyaluronic acids, fibers, silk proteins, fibronectins, alginates, agaroses, and chitosan are a few naturally occurring polymers and proteins utilized to create hydrogels.108 Numerous investigations have used hydrogels composed of natural polymers for the growth and culture of cancer cells. In a research study, the three different groups of cells MCF-7 of breast tissue, fibroblasts, including those linked to tumors, and myoepithelial cells were combined with a collagen hydrogel.109
The basement membrane comprises proteins for developing and structuring the tissues, including collagen IV, fibronectin, and laminin.110 For tissue engineering, mouse sarcoma cancer cultures of cells are frequently used to obtain the basement membrane (Matrigel). These membrane extracts comprise 30% collagen IV, 8% entactin, and 60% laminin, mimicking the natural environment. Additional growth factors and proteins, such as matrix-metalloproteinase, are present that alter the extracellular matrix.110 In contrast with cancerous cells administered subcutaneously by not using Matrigel, lung malignant cells mixed with Matrigel quickly expanded into a larger tumor mass when administered under the skin. When coinjected with Matrigel, A253, and B16F10, two additional carcinoma cell lines similarly demonstrated enhanced cell growth rates (five to ten times).111
It has recently been shown that non-invasive techniques like magnetic levitation can create sophisticated 3-D structures.112 Magnetically driven platforms have recently been modified and utilized in 3-D cell culture. It has been established that three-dimensional spherical tumors can be created using a magnetic cell levitation technique.113 Poly (L-lactic-acid)-b-poly(ethylene-glycol)-folate scaffolds or poly (lactic-co-glycolide)-encapsulated magnetic iron oxide (Fe3O4) were used in 3-D tumor cultivation of cells. A similar technique created a 3D culture of human glioblastoma cells.114
Polymeric scaffolds
Polymeric scaffolds resemble hydrogels as they are highly interconnected microporous structures. The primary distinction between hydrogels and polymeric scaffolds is that the former can have cells mixed with polymers before hydrogel formation, whereas the latter requires synthesis before cell seeding.115 Polymerized scaffolding originates from organic (Chitosan, Alginate, & Collagen) and artificial (PEG, PLA, PLG, PGA, PS, and PLGA materials.116 Commercially, a variety of polymeric scaffolds are offered. In labs, 2-D cell culture is frequently conducted using polystyrene culture plates. The benefits of 3-D topography can be obtained by synthesizing a scaffold based on 3-D polystyrene. According to one study, HBL-2 cells isolated from lymph nodes grew more quickly in a 3D PS scaffolding culture.117
Cancer-on-chip-based tumor models
Microfluidic technologies are widely used in medicine and disease diagnostics because they provide exact command over small amounts of liquid within the passageways.118 Additionally, several dynamic systems built on microfluidic technology are being developed to simulate the tumor microenvironment in vitro.119 Microfluidic platforms can study the impacts of torsion stress on cancer cells and provide growing cells with a constant supply of nutrients. Instead of living in empty surroundings, tumor cells are found in intricate scaffolds made of extracellular matrix. The creation of multi-aperture scaffolds presents far stronger advantages over spheroid cultures, including establishing tumor vascular systems in situ, low sample specifications, and rapid operation. The fundamental constituents of the cancer-on-a-chip comprise the microfluidic chips, structural substance, equipment for controlling flow, and elements of cells.120 Cancer-on-a-chip technologies have significantly advanced tumor biology, encompassing drug screening, cancer metastasis, cancer motility, and epithelial-mesenchymal transition. Several strategies have been implemented to achieve this goal, primarily involving soft lithography and microfluidic devices.
Soft lithography
Since 1988, advances in soft lithography have led to the development of nanocontact printing, which makes it possible to create scaffold nanostructures. Soft lithography is the initial step towards obtaining the master mold created by lithography. After that, the polydimethylsiloxane (PDMS) precursor is poured into the master molding. PDMS is biocompatible and has no toxicity, and any leftover bubbles are removed by vacuum degassing.121 It is finally cured by baking in order to cross-link the sample. A 7-channel microchannel plate was developed by Lee et al., which demonstrates how effective it may be in researching resistance to medicines and EMT.122
Microfluidic devices
Building biological scaffolds using cancer-on-a-chip models has garnered significant interest in recent years. Although tumor cells may be introduced into the mid zone, this microfluidic equipment regulates the development of chemical gradients between tubes with two access points: one facilitates the intended chemical passage, and the other provides a buffer.123 Researchers have proposed a three-dimensional tri-cultivation in chip equipment for pharmaceutical assessment and evaluation.124 Another compartmentalized device using a collagen extracellular matrix with a vessel-like path was created by Acosta et al.,125 The interstitial flow pressure gradient may impact the cell aggregates of a lumen plate used for seeding MDA-MB-231 cancer cells.
Limitations associated with the associated models
Certain limitations needs to be considered by the researchers before adopting the model in to their work. Spheroids have size related issues as the heterogeneity and variability in their development makes it difficult to interpret results accurately. The diffusion of nutrients and waste products becomes increasingly limited as their size grows. Spheroids still lacks the ECM environment and complexity of native tissue. Incorporating multiple cell types and other components poses another challenge.
Bioprinting holds immense potential for revolutionizing medicine but it is currently limited by challenges related to the complexity of tissues, material properties, resolution, cell viability, scalability, ethical considerations, cost, and long-term stability. Creating functional vascular networks within printed tissues, absence of optimal biocompatible inks with appropriate mechanical strength remains a significant challenge. The cost of bioprinting equipment, bioinks, and the necessary cell culture and maturation facilities can be prohibitively expensive. This limits access to bioprinting technology to well-funded institutions and slows down the widespread adoption of the technology. While microfluidic devices offer numerous advantages, such as reduced reagent consumption, rapid analysis, and the ability to perform complex tasks on a small scale, they also come with several limitations. Microfluidic devices often require precise fabrication techniques which can be time-consuming and expensive. These processes require specialized equipment and expertise, making them less accessible to researchers without the necessary infrastructure. The choice of materials for microfluidic devices can be limited. Finding materials that are biocompatible, durable, and easy to fabricate can be challenging, and scaling up production for commercial applications can be difficult and costly. Overcoming these limitations requires ongoing research and development, as well as collaboration between engineers, material scientists, and biologists to optimize the design, functionality, and scalability of microfluidic devices.
Tumor cell sensing and signal transduction
Cancer development and progression rely heavily on tumor cells’ ability to perceive and react to cues in their surroundings. These signals can be transmitted through various mechanisms, including volatile organic compounds (VOCs) that can travel long distances between cells.126 Tumor cells can also acquire mutations and epigenetic changes that disrupt signaling pathways controlling cell growth, division, and movement. These alterations can activate oncogenes and inactivate tumor suppressors, ultimately developing key cancer hallmarks.127 Stromal cells within the TME can secrete growth factors and chemokines that activate oncogenic pathways in cancer cells.128 This intricate reaction initiates a cascade of mechanotransduction pathways, where physical cues are converted into biochemical signals that impact cancer cell proliferation and migration129 (Figure 4). A thorough understanding of tumor cell sensing and signal transduction is crucial for cancer diagnosis, prognosis, and the development of targeted therapies. The mechanical responses are detected and triggered by activating mechanosensors, such as integrins, focal adhesions, and caveolins, and are studied in the following sections in detail:
Cell membrane protein receptors
Integrins
Integrins (cell surface receptors) link the ECM and cytoskeleton, sending the biological signal to related cells. Integrins can also change the protein structure and function in reply to physical signals from the tumor microenvironment (TME). Integrin structure is heterodimeric, with multiple subunits linked by non-covalent forces130 There is a place for ligands to bind within the domains, and multiple investigations have demonstrated that integrins have diverse levels of binding affinity.131 Integrin activation powers mechanotransduction and can allow proteins to enter into cells. One mechanism includes proteins attaching to cytoplasmic tails from inside the cell, whereas the other involves multivalent ligands from outside.
When subjected to stress in the changing mechanical microenvironment, integrins are stretched, activating and establishing a connection between the dynamics of ligand-receptor interaction and the mechanical control over conformation.132 More specifically, ECM fibrils can pull on ligand-coupled integrins with a force. Instead of directly adhering to actin, integrins use an adaptor protein (such as vinculin, talin, zyxin, and actinin) to transport these signals from the membrane to the cytoskeleton system. These adaptor proteins and integrinscan transmit actin-induced stresses to the extracellular matrix.133 ECM rigidity, a vital mechanical microenvironment component, is passive and difficult for cells to sense. Nevertheless, the rigidity can be identified through changes in distortion and the interplay between the actin and integrins. This is because the force exerted by cells during contraction varies according to the surrounding matrix.
Focal adhesions (FA)
These complex structures in the cell membrane interact with the ECM through integrins. They attract various associated proteins, forming physical interactions with the cytoskeleton.134 Focal adhesions undergo maturation in response to mechanical signals in the tumor microenvironment. It has been reported that mature FAs consist of over 180 proteins forming integrin adhesives. These proteins include vinculin, paxillin, talin, zyxin, tensin, and actinin.135 The receptor binding with the ECM causes focal adhesions under mechanical pressures. Many adapter proteins can help FA progenitors form “nascent adhesions”.136 Mechanical stress, such as cell contractility, increases actin bundle levels. This matures nascent adhesions into focal adhesions that allow cells to perceive the mechanical signals in the microenvironment.137 Based on extensive experimental data, FAs connect the cells with ECM by altering outside-in and inside-out signaling. The stiffness and structure of this ECM can affect the cellular placement, size, and spatial and temporal dissemination. Focal adhesions also regulate cytoskeletal structure via integrin-linked mechano-transduction. They control cellular shape, growth, invasion, treatment resistance, and gene expression.
Caveolins
Caveolae are cell-membrane receptors characterized by small omega-shaped invaginations, and these are known to be associated with several human disorders. They are commonly recognized as mechanical sensors and are crucial in numerous cell signaling pathways.138 Caveolae and lipid rafts vary primarily in the presence of certain membrane proteins called caveolins.139 Cav-1 and Cav-2 are two major types having high expression in several cellular types.
Caveolins, acting as mechanosensors, detect mechanical signals in the TME through processes such as flattening, disintegration, and phosphorylation of Cav-1 at position Y14. Sinha et al. said that the extension and disintegration of caveolins is a biological reaction to mechanical stress that does not need actin or ATP.140 Following the reduction through membrane tension, Cav-1 is liberated, enhancing movement at the cell surface. Caveolar endocytosis is initiated via modifications in the tension in order to control anchorage- dependent signaling. The protein component becomes more active upon caveolar disintegration. According to the findings, Cav-1, which has separated from its original structure, connects with a binding factor called BFCOL1, promoting the deposition of extracellular matrix.141 Cellular area is a significant mechanical determinant that influences cell activity. This can be modulated by mechanical signals that govern the levels pY14Cav-1, which impacts the organization and signaling of focal adhesions.142 Cav-1 can modulate the activity of YAP via regulating actin polymerization in response to alterations in ECM stiffness.143 Furthermore, the presence of mechanosensitive Cav-1 is essential for cellular invasion. It can also be triggered by shear stress that can stimulate several cellular processes, including motility, adhesion, invadopodia development, resistance to anoikis, and metastasis. Figure 5 represents how integrins and focal adhesion molecules play crucial roles in mechanotransduction pathways and tumor cell polarisation.
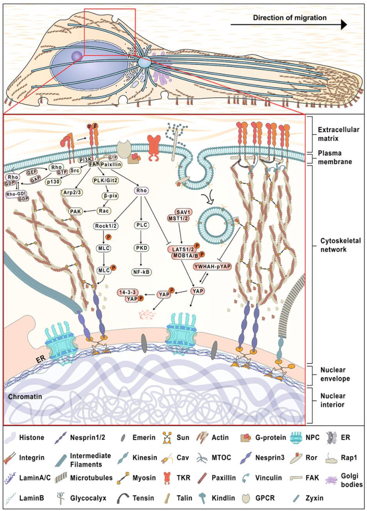
Figure 5 Mechanotransduction pathways and tumor cell polarisation, including integrins and focal adhesion. Reproduced with permission from.58 Copyright 2022, John Wiley and Sons.
Cytoskeletal mechanics
sSeveral mechano-sensors, including integrins and focal adhesions, have demonstrated that they establish linkages with the cytoplasm actin. These studies have also indicated that actin filaments are mechanosensors, detect the tension applied over cells, and trigger subsequent signaling cascades. The cellular skeleton comprises actin, microtubules, and intermediate filaments, each having distinct functions. Filamentous (F-actin) and globular (G-actin) actin comprise the actin framework, which undergoes dynamic assembly and disassembly.144 When attached to rigid surfaces or extensive nanopatterned surfaces, the proportion of these two proteins changes, forming stress fibrils. This triggers overexpression of YAP, thereby controlling the transduction through the Hippo pathway.
Consequently, combining YAP with the TEA/ATTS protein promotes the expression of target genes, facilitating their activation. This phenomenon has been observed through experiments and is documented in scientific literature.145 YAP is associated with the evolution of malignancy and is mediated by the acting mechanical forces and actin dynamics. Furthermore, the relaxed actin filaments enhance the binding of cofilin, which in turn promotes the disintegration of actin filaments.146 Actins, functioning as mechanosensory, collaborate with myosin, generating certain contractions and propelling the cell forward. Cancer cells utilize this process to respond to applied strain in the surrounding matrix.147 Microtubules are vigorous heterodimers of various tubulin components. They have certain important functions in cell growth, development, and vesicle transport. Microtubules (an important part of the cilia axoneme) detect and convert several mechanical signals from the external environment.148 Microtubules also serve an important purpose in facilitating the spindle’s organization, chromosomes’ alignment, and segregation during mitosis.
Integrin- FAK signaling
Recent research examinations have shown the importance of integrins in adhesion, proliferation, and migration by facilitating bidirectional communication.149 FAK regulates the outside-in signal transduction, which uses several signaling molecules. Mechanical inputs can enhance the integrin binding to the extracellular matrix, activating FAK through conformational changes. Later, FAK activates Src, which binds with p130 CRK-associated substrate (p130CAS) and interacts positively. FAK progressively activates (PKL/Git2), transmitting signals via Rac and p21-activated PAKs.150 These pathways are similarly affected by integrin-FAK as they facilitate cell entry via mechanical stimulation. Kindlin and Talin control inside-out integrin signaling. Talin recruited by RAP1 binds cell surface integrins via Rap1-GTP-interacting adaptor molecule.151 Kindlin initially activates the integrin β-subunit and later employs paxillin to activate Rac1. Its connection to the Arp2/3 junction assists rac1-induced membrane protrusions.152
Rho signaling
Around 22 Rho-family components switch between GTP and GDP-bound states. Cdc42, Rac1, and RhoA are the most researched Rho GTPases that rebuild the cytoskeleton by regulating downstream protein activity.153 ROCK1, ROCK2, and RhoA/RhoC control the cytoskeleton, influencing actin depolymerization and myosin contractility by targeting LIMK. ROCK strongly phosphorylates MLC2 and LIMK, which increases actin formation and myosin contraction.154 Mechanical cues in the tumor microenvironment control guanine exchange factors and Rho GTPase activators by controlling the remodeling of the cytoskeleton.155 Yang et al. showed that p190RhoGAP temporarily blocks RhoA and rearranges actin proteins in response to shear stress. This is achieved by the phosphorylation of p190RhoGAP by Src, which occurs in the mechanosensor Cav-1 and integrin pathway.156
Hippo signaling
This pathway has been well-conserved throughout evolution and contains transcription factors and protein kinases involved in tissue regeneration, cell proliferation, wound healing, organ development, and apoptosis.157 Recent studies have linked the Hippo pathway to tumor formation and cancer dissemination. Mechanical signals, including stiffness and shear stress, can activate the Hippo pathway in the tumor microenvironment governing cellular polarity.158 YAP/TAZ, crucial transcription cofactors, get a phosphate group from MOB1, allowing it to be sequestered in the cytoplasm, thus preventing its translocation into the nucleus and impairing its transcription cofactor function.158 However, unphosphorylated YAP/TAZ often translocates into the nucleus and connects with transcription factors such as TEADs and RUNX families, ultimately controlling cell migration and invasion.
Nuclear mechanics
The nucleus can react to mechanical variables by its physical link with the cell membrane through the actin cytoskeleton. It acts as a cellular information source and plays an essential part in the transmission of signals in both directions.159 The linker of the nucleoskeleton and cytoskeleton (LINC) carries the mechanical signal from the cell membrane to the nucleus. Mechanical stresses can change nuclear structure, chromosomes, gene location, and expression through LINC complexes.160 These contain a characteristic KASH domain on the outer membrane and a SUN domain on the inner nuclear membrane. Nesprins, KASH, and lymphocyte-restricted membrane proteins are mammals' six KASH proteins and SUN proteins coordinating lamins and chromatin. Research suggests a full mechanotransduction pathway may send signals from the extracellular environment to nucleus DNA. The actin connects with the nuclear membrane via nesprin and interacts with KASH to connect the lamin and DNA. 161
In contrast, LINC complexes aid inside-out signaling in several ways. Sun2 activates RhoA and increases cell focal adhesions. Sun1 restricts Sun2 activity, preventing stress fiber production. FHOD1 inhibits the ROCK-based actin modulation, further restoring the aberrant cell shape. LINC complexes contemporises the cell-cycle advancement by mechanically stimulating the chromatin regulator factor.162
Nuclear lamins
These comprise a thick network of intermediate filament proteins beneath the nuclear membrane. It helps sustain the nuclear structure and is implicated in cell relocation, nuclear position, chromatin architecture, and DNA synthesis.163 The LMNA gene produces lamin A, C, A10, and C2. The second group consists of B-type lamin produced by the LMNB genes and is consistently expressed in all cells.164 Lamins are situated near the nuclear envelope and establish interactions with most of the proteins involved with the nuclear membrane. This interaction serves to provide a mechanical support system. Anchoring proteins, A-type lamins, are involved in mechanotransduction and chromatin reorganization. Cells with lamin A mutants do not conduct the cell’s mechanical stresses, thus concluding a direct association between matrix stiffness and lamin A protein levels. On a soft matrix, low lamin A levels promote adipogenesis, while high levels on a rigid matrix promote osteoblast differentiation.165 Lamins control the mechanotransduction pathway transcription factors and signaling molecules. As transcriptional regulators, lamins influence signaling pathways via c-fos or Notch.166
Chromosome reorganization
Alterations in chromatin structure activate or deactivate genes to regulate protein expression. Force-induced nuclear deformation alters the chromatin architecture that can activate or suppress multiple genes.167 It can force the cell membrane to detach Cajal bodies from chromatin. In 5 seconds, actin cytoskeleton remodeling occurs and depolymerizes the chromatin network. Actin transfers force better than microtubules.168 The mechanical stimuli affected these regions, and the applied force also influenced the binding of core histone H2B, methylation, acetylation/deacetylation, and phosphorylation. Studies using topographic patterns and non-uniform mechanical stress showed the importance of histone acetylation in controlling gene expression.169 It was also shown that chromatin remodeling changes the epigenetic markers and activates Wnt-responsive gene transcription. The binding proteins of nuclear actin can also attract histone remodelers to transcription sites and alter gene expression.170
Role of mechanosensing in developing chemotherapeutic resistance
Cancer cells have developed multiple drug resistance strategies. Here, we cover different mechano-resistance pathways based on preclinical and clinical data. Cancer cells can repair DNA damage and remain one of the ways by which chemotherapeutic resistance is induced.171 In ovarian and lung cancer, increased DNA repair signaling that correlates with cisplatin resistance and increased cancer survival is observed.172 One such gene family, ATP-binding cassette (ABC), can bind and efflux out the drugs. P-glycoprotein is another efflux pump that reduces the effectiveness of chemotherapy and increases ovarian cancer survival.173 Several signaling pathways, mainly PI3K/Akt and p38-MAPK that regulate this protein expression have been identified. Higher Akt protein levels are connected to ovarian cancer resistance against paclitaxel and cisplatin and gastric cancer resistance towards doxorubicin.174 Various checkpoints control cell cycle progression and the cells that can stop the cell cycle might be less susceptible to chemotherapy. Genetic defects in the cell or environmental conditions can trigger chemotherapy resistance pathways175 (Figure 6).
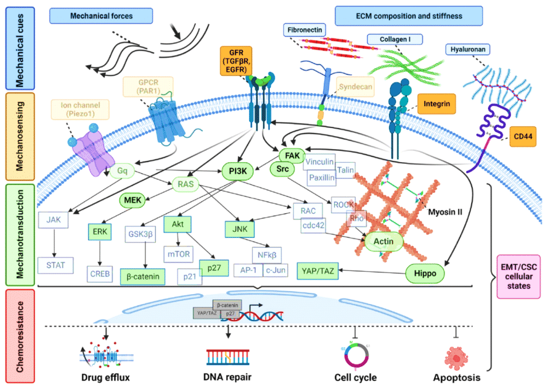
Figure 6 Mechanical stimuli and cancer cell chemoresistance systems share signaling responses. Reproduced with permission from.172 Copyright 2023, Elsevier.
The changes in ECM cause desmoplasia and dynamic stiffness alterations. Thus, increased stiffness will aid tumor blood vessel growth, cell transition between epithelial to mesenchymal states, and tumor spread.176 Rice et al.,176 found that increased matrix stiffness promotes epithelial-mesenchymal transition, resulting in a mesenchymal phenotype and paclitaxel resistance.177 Many cancer models show that microenvironment stiffness increases resistance to conventional and tailored chemotherapies.178 Therefore, controlled stiffness models are needed that can precisely represent cancer drug resistance. 3D bioprinting permits using various materials, their composition, and cross-linking to control structural stiffness. The stiffness might change with degradation in certain bioinks, such as alginate.179 Monferrer et al., created stiffness gradients using GelMA and different AlgMa concentrations. These gradients showed how intercellular space stiffness affects neuroblastoma clinical behavior in cancer research.180 Figure 6 represents the similar signaling responses between cancer cell chemoresistance systems and mechanical stimuli.
Novel drug development requires in vitro research before preclinical research. 3D-bio printed models might improve preliminary high-throughput drug screening and target candidate selection by yielding fast and reproducible results.181 It also allows the production of disease models for pharmacological selection. A 3D-bioprinted structure drug was created with cholangiocarcinoma cells.182 The study found that these bio-printed cells behaved like stem cells and were resistant to numerous drugs compared to two-dimensional cells. Later, a fibrin bioink was used to generate glioma cell clusters, affecting the reactivity to novel glioblastoma treatments.183
Signal transduction pathways in drug resistance
Integrin signal transduction pathways are important in making cancer cells radiation- and chemotherapy-resistant. The changes in the DNA repair mechanism via matrix-engaged β1 integrins impart radiation resistance in different carcinomas.184 Temozolomide-resistant glioblastoma cells are connected to α5β1 integrin signaling that suppresses the p53 pathway.185 ERK stimulation by α2β1 takes control to provide resistance towards doxorubicin. Syk tyrosine kinase plays a role in signaling pathways of lymphocytic leukemia, involving α4β1 binding by VCAM1 and CXCR4. Thus, Syk inhibition boosts leukemia cell susceptibility towards fludarabine, suggesting that chemotherapy and Syk inhibitors play a role in leukemia.186 Integrin-dependant chemotherapeutic resistance varies by tumor type and drug mode of action.
Recent experiments have demonstrated the integrins’ role in molecular targeted drug resistance. Lapatinib and trastuzumab are more effective against ErbB2-positive breast cancer cells when the laminin-binding integrins α6β4 and α3β1 are blocked in vitro. Additionally, FAK and SRC are upregulated in breast cancer cells for their ability to withstand the action of both drugs.187 Inhibiting FAK with certain drugs can greatly reduce drug-resistant cell proliferation in 3D Matrigel. These data suggest that FAK or SRC kinase inhibitors may improve ErbB2 therapy. According to a study, PIK3CA mutations in organoids derived from an ovary cancer cause the basement membrane to adopt adaptive methods that avoid targeted therapy.188 In matrix-attached cancer cells, it was found that FOXO and CAP control the expression of IGF1R, EGFR, and ErbB2. This change can make the cell resistant to PI3K and TOR inhibitors. Multiple studies have shown a complicated signaling network that induces drug resistance. Inhibiting the FAK and deactivating NF-κB in endothelial cells decreases the cytokine levels and boosts resistance towards doxorubicin.189 In squamous cell carcinoma models, FAK removal or inhibition restores cancer-fighting ability. Pancreatic cancer mice with FAK inhibition developed less desmoplastic stroma. This activation changes matrix structure and stimulates β1/FAK/SRC signaling in melanoma cells.190 In some therapeutic contexts, FAK inhibition may reduce medication resistance by targeting integrin-mediated intercellular connections.
Mechanosensing crosstalk and anomalies inducing drug resistance mechanisms
Cell membranes physically separate internal components from the outside world and are essential for mechanical stimulus modulation. Many cell-membrane integrated proteins transform these mechanical inputs into internal signals (Figure 7). EMT cells have shown an upregulation of genes that pump drugs out of the cell, resistance to programmed cell death, anoikis, and stem cell-like traits.191 Slow cell division, high pro-survival mechanisms, increased drug ejection, and DNA repair can make the CSCs chemoresistance.192 Herein, we examine how different cell states and mechanical defects affect the chemoresistance and chemotherapy response. Integrins, GPCRs, and ion channels sense the change in mechanical stresses around the tumor microenvironment.
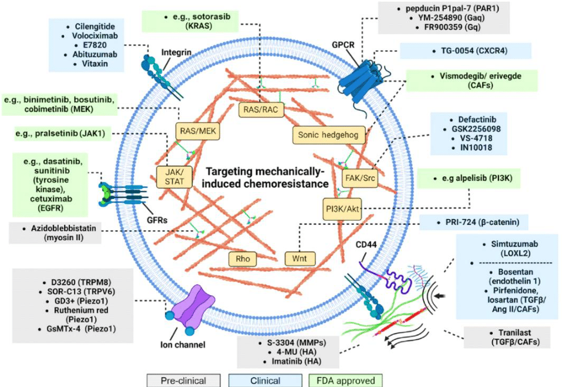
Figure 7 Effect of various drugs towards mechanically induced chemoresistance. Reproduced with permission from.172 Copyright 2023, Elsevier.
Meanwhile, mechanosensors regulate the receptors associated with RAS, PI3K, FAK, and Hippo signaling. These mechanisms can improve and re-modulate cytoskeleton arrangement and activate ROCK/Rho, RAC, and myosin pathways. It also controls the nuclear travel of certain factors that regulate protein upregulation. These mechanically generated responses can stimulate drug ejection, DNA repair, cellular proliferation, and programmed cell death, thus forming the four major chemoresistance pathways.193 Integrins, Piezo, TRP, and GPCRs are common mechanical signal receptors on cell surfaces. Ion channel gates are opened and closed under mechanical load, and certain ECM components can indirectly cause this reaction. Ion channels and focal adhesions govern cellular reactivity by detecting mechanical stresses. Mechanical signals activate multiple ion channels in breast cancer cells, increasing metastasis risk.194 In some malignancies, overproduced GPCRs can cause cell growth in response to signaling molecules or physical stimuli.
Multiple sources have examined how this cell response impacts tumor development and treatment resistance.195 Mechanical signaling components can be inhibited and normalized to modify signaling pathways. Phase 1 and 2 clinical trials of integrin inhibitors showed promising results.196 However, combining chemotherapeutics with pathway inhibitors has demonstrated no survival benefit. The mechano-sensors aberrantly affect the mechanotransduction pathway that restores chemical stimulus sensitivity. Piezoelectric ion channels and transient receptor potential (TRP) are common therapeutic targets.197 It was shown that suppressing the TRPC5 pathway can improve adriamycin lethality, while TRPM2 is associated with doxorubicin and tamoxifen resistance.198
Figure 7demonstrates the Impact of different medications on mechanically produced chemoresistance. Furthermore, a 20-GPPD, ginseng saponin derivative, activates the TRPC channels that allow calcium ions to enter, resulting in colon cancer cell death. Blocking TRPC6 and lowering Ca2+ levels can halt gastric cancer cells.198 These early clinical trials have mechanosensitive ion channel TRP activators to provide promising results.
Mechanobiology is now in an excellent position to solve important problems associated with different cancer kinds and classifications. Our review emphasized the importance of mechanical variables in cancer formation, drug resistance, and state of dormancy in the metastatic cascade. These processes are regulated by well-characterized pathways that offer potential therapeutic targets. Future research could explore strategies to manipulate the abnormal tumor microenvironment to enhance drug delivery and improve response to chemotherapy. More biomimetic models of tumors will be necessary for these investigations to understand how cancer cells perceive their surroundings, interact with fibroblasts linked to malignancy, and respond to mechanical stress. By incorporating these aspects, researchers can design more effective therapeutic strategies to combat various cancers. The significance of considering mechanical domains in the development of tumors is reinforced by altered ECM rigidity. Model systems replicating several stages of the metastasis cycle are useful because cancer-associated and malignant fibroblasts have mechanical memory. These models can be employed to study how cancer cells respond to mechanical stress during adhesion, intravasation into blood vessels, and even colonization of new sites, providing crucial insights into how tumors navigate the complex mechanical challenges throughout the metastatic cascade.
Furthermore, studying the mechanical domain could help with cancer classification and more precise prognostication for drugs that target mechanotransduction pathways. Separate cell lines and identically immunologically profiled cells differ significantly in mechanical response. Mechanotyping, including measurements of traction force, adhesion profile, nuclear deformability, and cytoskeletal stiffness, could improve present categorization techniques and assist in resolving the system’s heterogeneities. In a comparatively short time, morphological structure and movement assessment on different topographies can be used to understand the aspects of cell activity. This knowledge holds promise for a more efficient and specific approach to cancer diagnosis and treatment.
Similar to how blood flow properties can reveal cell health, microfluidic analysis can rapidly identify cell types and abnormalities by passing blood samples through tiny channels and analyzing their mechanical response. One possible approach would be to try dynamic typing through mechanical loading regime response. Major mechanotransduction regulators activated by mechanical loading would augment surface marker techniques. Building high-throughput mechanical loading systems for these tests could be necessary. To elaborate, a novel tool that combines compression, fluid shear, stretch, and different loading regimes will be helpful in the study of tumors. The simple loading regimens used by current technologies may not accurately represent the complex and dynamic mechanical loads present in vivo. Overall, this comprehensive approach could lead to a powerful new tool for cancer diagnosis and unlock a deeper understanding of tumor mechanobiology.
The Prime Minister’s Research Fellowship supports N.J. Other authors declare that no financial support was received for this article’s research, authorship, and/or publication.
Credit authorship contribution statement
N.J.: Conceptualization, Literature collection, Writing - original draft, Writing - review & editing, Validation, Visualization, Figure Preparation, Formal analysis. Y.O.W.: Literature collection, Writing - original draft, Writing - review & editing, Formal analysis. S.D.: Writing - original draft, Figure Preparation. V.V.: Writing - review & editing. S.D.: Writing - original draft, Validation, Visualization, Writing - review & editing, Figure preparation. All authors read and approved the final manuscript.
Data availability
No data was used for the research described in the article.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
The authors declare that they have no conflicts of interest.
None.

©2024 Jain, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.