eISSN: 2379-6367


Case Report Volume 7 Issue 5
1Department of pediatrics, University of Cuenca, Ecuador
2Department of Imagenology, University of Cuenca, Ecuador
Correspondence: Manolo Patricio Maestre Calderon, Department of pediatrics, University of Cuenca, Ecuador, Tel 0984883156
Received: August 19, 2019 | Published: September 12, 2019
Citation: Calderon MPM, Robles ACR, Astudillo MEL, et al. Malformation of Abernethy type 2: presentation of a clinical case and review of literature. Pharm Pharmacol Int J. 2019;7(5):209-212. DOI: 10.15406/ppij.2019.07.00253
The extrahepatic congenital porto-systemic derivation is a very rare pathology of the venous vascular system of the abdomen described by John Abernethy in 1793. In this malformation there is a passage of substances not metabolized from the liver to the systemic circulation and dilation of the pulmonary capillary bed. It is divided into 2 groups according to the presence (type II) or total absence (type I) of portal flow. We present the case of a 6-year-old child, a twin product of the first pregnancy of a 22-year-old mother, attended at Vicente Corral Moscoso Hospital in the city of Cuenca, with respiratory symptoms without definitive diagnosis. The presence of a portal vascular abnormality was found in a casual abdominal ultrasound study diagnosed as Abernethy type II malformation by abdominal angiography and nuclear magnetic resonance. A thorough bibliographical review and a subsequent discussion of the case were carried out; finding as important data the existence of 80 cases reported until 2014 worldwide. In Latin America there is the report of a case in Venezuela in 2011 and in Argentina in 2016. We conclude by emphasizing the importance of a correct anamnesis, physical examination and the use of different diagnostic techniques in terms of respiratory symptoms due to there are rare pathologies that once an early diagnosed they can be treated successfully, thus giving the patient a normal life. The present case is the only one reported in Ecuador with a successful approach and treatment.
Keywords: malformation, portal vein, pulmonary hypertension, extrahepatic, congenital abnormality
Abdominal venous system malformations are rare vascular alterations that occur during the embryonic period after the formation of new venous systems. They are generally associated with other malformations, among which are mainly portocava anastomosis and venous duct agenesis with asymmetric cardiac development and intestinal rotation. These anomalies have been described occasionally associated with chromosomal alterations such as trisomy 21.1 The first described case of an Extrahepatic Congenital Portosystemic Shunt (SPCE) was in the year of 1793 by John Abernethy. In 1883, Kiernan described the second case of congenital porto cava anastomosis in an 18-year-old adolescent in whom the hepatic artery was elongated.2 This malformation is classified into two variants according to its anastomosis with the portal vein and its anastomosis with the inferior vena cava (IVC). In type I there is a complete deviation of portal blood to the IVC and the extrahepatic portal vein is absent; while in type II there is a partial portal flow to the liver through a hypoplastic portal vein (VP).Since its discovery 80 cases have been described until 2014 worldwide, the majority of patients under 18 years. Type I malformation occurred in 74% of the reported cases, is present in childhood and is associated with other malformations. Until 2013, 32 cases of type I were reported worldwide.
Abernethy Type II malformation occurs more frequently in adulthood and is more common in male patients, is associated with liver dysfunction and encephalopathy.1,3,4 Patients with Abernethy malformation have a wide variety of symptoms ranging from asymptomatic cases that are diagnosed in adulthood to severe hepatic manifestations at birth. Cases of premature puberty, hyperinsulinemia and hypothyroidism associated with this pathology have been reported.5 The diagnosis is mainly made with Doppler ultrasound. Computed tomography angiography and magnetic resonance angiography are used for subsequent shunt classification and evaluation of associated abnormalities.6 The treatment will be planned according to the type of referral. Patients with Type 1 Abernethy Malformation need clinical, biochemical, imaging follow-up, with liver transplantation being the only treatment; while Type 2 malformation requires the early closure of shunt or embolization to resolve hypoxemia and prevent hepatic encephalopathy.3,7
Male patient, 6 years old, referred to Vicente Corral Moscoso Hospital (HVCM) in Cuenca for presenting distal cyanosis, dyspnea, asthenia and hyporexia since 2014. At 3 years of age in Quito he is diagnosed with cystic fibrosis by positive sweat chlorine test (119 mmol/l), with subsequent treatment. Having no improvement, a genetic test was performed 6 months later with a negative result, thus suspending the treatment for cystic fibrosis. During 2015 the patient remains asymptomatic in the respiratory sphere. In 2016 he presented the same symptomatology again, so he was hospitalized with a diagnosis of pneumonia, being discharged with home oxygen. Due to the constant exacerbations of respiratory symptoms, it refers to a third level Hospital, HVCM, where its admission is decided in 2017 for a diagnostic approach. Important background: Twin pregnancy product of a 22-year-old primigent who attended with a threat of preterm birth at 24 weeks gestation. Patient is born by caesarean section after fetal distress, is admitted to neonatology for presenting respiratory distress at birth, remained in non-invasive mechanical ventilation for 2 days and is discharged at 48 hours in better conditions.
Symptomatology
2014-2017: peripheral cyanosis, dyspnea, asthenia and hyporexia.
Physical exam
Vital signs: heart rate: 78 per minute, respiratory rate: 32 per minute, blood pressure: 102/66 mm/Hg, temperature: 36.5°C, oxygen saturation: 78%, FiO2: 21%. Weight: 16.5 Kg (Low weight for age according to MSP tables), size: 113 cm. Skin: generalized paleness, venous network in thorax and abdomen, capillary filling less than 2 seconds, mild facial telangiectasias. Chest: GIII holosystolic murmur. Abdomen: soft, depressible, not painful, no visceromegaly, hydro-noise present. Tips: digital hypocratism. Neurologically without alterations (Figure 1).
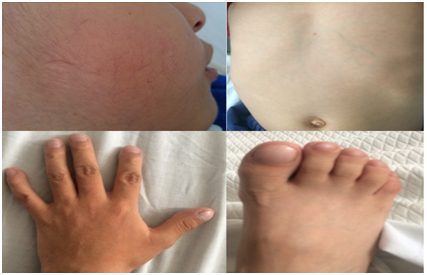
Figure 1 A, Facial telangiectasia; B, Venous network in thorax and abdomen; C, Digital hypocratism in hands and feet.
Presumptive diagnoses
Chronic lung disease, chronic heart disease, liver disease, pulmonary arteriovenous malformation, hepatopulmonary syndrome, primary pulmonary hypertension, Abernethy malformation.
Diagnostic procedures
A contrasted echocardiogram was performed that recorded the passage of bubbles to the left cavity within 3 seconds of placing the contrast, the entire interatrial and interventricular septum, suggestive of extracardiac fistula.
During his hospitalization he presents nonspecific abdominal pain, so abdominal ultrasound is performed, where he reports at the vascular venous level, dilated superior mesenteric vein of 7.4mm, vascular venous anomaly with an approximate diameter of 1.17cm, which originates at the confluent mesenteric level. Splenic and runs through the abdomen in a caudal direction, with hepatofuge flow, leading to the level of the common left iliac vein; to consider congenital porto-systemic shunt, with recommendation of abdominal angiotomography. The angiotomography reported porto-cava communication, with anomalous drainage vessel with a 1.42 cm caliber, which goes from the spleno - mesenteric junction to the left iliac vein. Hepatic artery and its branches with normal diameter and distribution. Porta vein with 4.7 mm caliber, and hypoplasia of the left portal. Magnetic resonance imaging of the abdomen reported a tortuous vessel with a 12 mm caliber, which originates in the pelvic hollow, ascending in parallel and to the left of the abdominal aorta, reaching the splendid-mesenteric confluent, suggestive image of systemic porto-cava shunt type II (Figure 2 & 3).

Figure 2 A, Contrast echocardiogram: bubbles in left heart cavities; B, Abdominal ultrasound: 0.74cm superior mesenteric vein dilation; C, Anomalous vessel with dilatation of 1.17cm.

Figure 3 Angiotomography. A, Porto-caval shunt of 1.42cm; B, 4.78mm portal vein, hypoplasia of the left portal is observed.
Treatment
Pre-surgical: propranolol, fluticasone. Surgical: intervened on 06/19/17 performing identification of the inframenterental shunt, dissection, clamping and ligation were performed.
Post-surgical: ceftriaxone, clindamycin, omeprazole, phytomenadione, metamizole and enoxaparin. Due to severe pulmonary hypertension (81mm Hg) it was managed with sildenafil, spironolactone and propranolol plus oxygen by nasal tips at 2 liters with progressive weaning (Table 1).
July 30, 2014 |
Sweat chlorine test: positive (119mmol/l). Extra HVCM. |
December 11, 2014 |
Genetic test: negative for cystic fibrosis. Extra HVCM. |
February 9, 2016 |
Diagnostic cardiac catheterization: ruled out pulmonary arteriovenous fistula. Extra HVCM. |
May 15, 2017 |
Sweat chlorine test: negative (55mmol/l) in HVCM |
May 17, 2017 |
Chest x-ray: signs of pulmonary hypertension. |
May 18, 2017 |
Abdominal ultrasound: dilated anomalous vessel of 1.17cm and dilated superior mesenteric of 7.4mm. |
May 22, 2017 |
Abdominal angiotac: evidence of porto-cava communication with abnormal drainage vessel. |
May 23, 2017 |
Contrast echocardiogram: 38mm Hg lung pressure and left ventricle with good function. The passage of bubbles into the left cavity is observed 3 seconds after the contrast, the entire interatrial septum and interventricular septum. Suggestive of extracardiac fistula. |
June 19, 2017 |
Surgical correction of shunt and bronchoscopy: chronic tracheo-endobronchitis and bronchopulmonary suppuration. |
June 21, 2017 |
Liver ultrasound: preserved diameter portal, recanalization of the left portal with adequate flow is observed. Thrombus in the portal that does not produce stenosis. |
June 26, 2017 |
Portal hepatic Doppler ultrasound: normal flow of portal and splenic vein, thrombus inside the 16mm portal vein that does not affect the flow. No collateral veins are observed in the spleno-renal space. |
July 05, 2017 |
Abdominal Doppler ultrasound: permeable spleno-portal circulation, without endoluminal images. |
August 18, 2017 |
Hepatobiliary ultrasound: normal sized liver and echogenicity, no focal or diffuse lesion is observed. No thrombus was observed in the portal. The spleno-mesenteric junction is normal. Suprahepatic veins with normal spectral wave morphology. |
August 22, 2017 |
Color Doppler echocardiogram: severe suprasystemic pulmonary arterial hypertension (81mm Hg), mild dilation of right cavities and left ventricle with good function. |
Table 1 Important milestones
Evolution
Inpatient with a diagnostic approach to chronic lung disease versus heart disease. On admission, he presented distal cyanosis, dyspnea, asthenia and hyporexia, with oxygen needs saturating 86% with ambient air. In the laboratory report without leukocyte formula, or anemia, mixed hyperbilirubinemia (2.1mg/dl) with a predominance of the indirect one (1.4mg/dl), high oxalacetic glutamic transaminase (TGO: 71 IU/l) rest of liver function and Renal without alteration. HIV serology is negative, hepatitis A, B and C negative antigens, cytoplasmic antineutrophil antibodies (ANCA) and negative antinuclear antibodies (ANA). In arterial gasometry with hypoxemia (SO2 83.5).
After the tests performed, Abernethy type II malformation is confirmed, so it is surgically operated for ligation of the porto-cava shunt, finishing the procedure without any intraoperative complications. Subsequently admitted to the Intensive Care Unit for 4 days for surveillance and follow-up with favorable evolution. Patient with good general condition that in the post-surgical exams did not present leukocyte formula, liver enzymes within normal parameters without hyperbilirubinemia. In the control gasometry performed one hour after surgery, patient with oxygen saturation of 96.8%. Dopplerde control ultrasound showed thrombus in the portal that did not compromise the flow, so it was maintained with enoxaparin of low molecular weight. In the post-surgical echocardiogram he presented severe pulmonary hypertension (80mmHg), managed with sildenafil, spironolactone and propranolol. In subsequent Doppler ultrasound controls, he did not present intra-portal endoluminal images and the pulmonary pressure was normalized.
Abernethy or SPCE malformation is a rare condition in which portal blood is partially or totally derived in the systemic circulation due to abnormal communication between the portal system and the systemic circulation. First described by Jhon Abernethy in 1793 in an autopsy performed on a 10-month-old infant who died of unknown cause, with congenital absence of the portal vein, mesenteric-cava shunt, dextrocardia and transposition of large vessels. Howard and Davenport classify this malformation into two types. Type I Abernethy malformation where there is a complete derivation of the portal venous flow within the vena cava (VC), that is, the systemic circulation (end-to-end derivation), presenting two subtypes: Type I a-presents the congenital absence of the VP due to the lack of union between the superior mesenteric vein (VMS) and the splenic vein (VE) and Type I b-in which the VMS and VE form a common fluence or trunk, despite being present do not supplement the liver. This denomination of confluence as a portal could be correct since it contains portal venous blood. Abernethy type II malformation presents the intact intrahepatic portal vein, however, the venous flow is diverted to the vena cava through an extrahepatic shunt from side to side, it is more common in the male sex according to our case. Type IIa is congenital and acquired IIb, which covers several cases of cirrhosis.1,6,8
There is a variety in its clinical and symptomatic presentation as neonatal cholestasis, hyperammonemia (26% patients), hepatic encephalopathy (10-14% of patients), elevated liver enzymes, jaundice, abdominal mass, cyanosis, however, there are those who manifest cardiac or respiratory symptomatology such as pulmonary hypertension, respiratory distress, digital hypocratism, hypoxemia coinciding with the clinical presentation of the present case that in addition to this presented liver enzyme alteration. In 2016, in the Republic of Korea, a case of Malformation was reported of type II Abernethy in a 19-year-old man who presented only abdominal pain of 5 days evolution in the upper right quadrant of the abdomen. It should be noted that there may be subclinical conditions and that the patient remains asymptomatic for life.2,5,8,9 Kanamori in 2003 reported a case of type II Abernethy malformation in a 4-year-old girl with a giant liver mass, arterial duct persistence (PCA) and cerebral atrophy, signs that differ from this study.4
From an endocrinological point of view, there are no studies associated with this malformation, despite this a study carried out at the Marmara Turkia Hospital in 2014, reports 2 cases of Abernethy malformation associated with early puberty, hyperinsulinism, hyperandrogenism and hypothyroxinemia, data that were not investigated in this case since the patient's symptomatology was not suggestive of endocrinological alterations.5 One of the symptoms that attracts attention is chronic hypoxemia, present in our patient, exposed by the digital hypocratism he presented. Considering hypoxemia, pathophysiologically, a short circuit (shunt) from right to left would be considered, which in turn are divided into intracardiac and intrapulmonary, which were ruled out by echocardiography and diagnostic cardiac catheterization respectively. When performing the contrasted echocardiogram of bubbles, it was positive after its arrival at 3 seconds in the left cavity being suggestive of extracardiac fistula.10
Abernethy malformation is one of the causes of Hepatopulmonary Syndrome, which is characterized by the triad of liver disease, arterial hypoxemia and pulmonary vascular dilation, producing diffuse dilatation of the arterial, capillary and venous vessels at the liver level given by humoral factors from the splanchnic venous circulation that are normally metabolized in the liver, however, after the presence of an extrahepatic shunt, these substances pass directly to the systemic and pulmonary circulation. The hypothesis of these humoral factors are high: high level of circulating endothelin-1 throughout the body, increased angiotensin and translocation of bacterial endotoxins that in turn increase the amount of nitric oxide. These substances cause the pulmonary pre and post capillary vessels that normally measure between 8-15 microns in diameter to increase between 100-500 microns and decrease the vasoconstriction produced by hypoxia, giving the passage of partially oxygenated hemoglobin to the systemic circulation with the consequent hypoxemia.7,10,11
Early recognition of this malformation is very important, since the SPCE increases the risk of liver neoplasms, benign focal nodular hyperplasia, hepatocellular adenoma and regenerative nodules which can develop later. It is estimated that 50% of patients with SPCE have nodular liver lesions, with type I malformation associated with hepatocellular carcinoma and hepatoblastoma3,5 There are innovations regarding the treatment of this malformation. It is clear that for the malformation of Abernethy type I the definitive treatment corresponds to liver transplantation, while for type II the ligation, embolization of the shunt is performed, including the placement of aortic stenting within the IVC. It is interesting to mention the use of three-dimensional impressions, which have helped to develop accurate representations of the patient that help surgical and endovascular planning, as there are discrepant dimensions between computed tomography, conventional venography and intravascular ultrasound.6,12,13
The present case demonstrates the importance of a correct anamnesis, physical examination and use of the different diagnostic techniques in terms of respiratory symptoms, due to the fact that there are infrequent pathologies that oblige us to analyze their origin very carefully, since not always they will have a pulmonary origin, but extrapulmonary as in our case. It is very important to familiarize yourself with the main clinical characteristics and the main diagnostic techniques of Abernethy malformation, since its early recognition will allow us an optimal clinical or surgical management, allowing to reduce or completely inhibit the symptoms, which will positively influence the quality of the patient's life.
None.
Authors declare that there is no conflict of interest.

©2019 Calderon, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.