eISSN: 2379-6367


Research Article Volume 6 Issue 4
1Department of Pharmacology, AR College of Pharmacy & GH Patel Institute of Pharmacy, India
2Department of Pharmacology, Indukaka Ipcowala College of Pharmacy, India
Correspondence: Varsha J Galani, Department of Pharmacology, Indukaka Ipcowala College of Pharmacy, New Vallabh Vidyanagar-388121, India , Tel 91-9429161203
Received: May 17, 2018 | Published: July 10, 2018
Citation: Darji PH, Galani VJ. Investigation of monoaminergic system mediated antidepressant action of Musa paradisiaca using various experimental models. Pharm Pharmacol Int J. 2018;6(4):287-292. DOI: 10.15406/ppij.2018.06.00188
Musa paradisiaca Linn, is one of the popular and important medicinal plants of India. Musa paradisiaca (Linn.) has several therapeutic applications in folk medicine in managing wide range of disorders including nervous disorder (nervine tonic). The effects of antidepressant treatments have traditionally been discussed primarily in terms of effects on noradrenergic, dopaminergic and serotonergic systems. Based on this investigation of monoaminergic system mediated antidepressant activity of Musa paradisiaca fruit was carried out using various experimental models. 14 days treatment of hydroalcoholic extract of Musa paradisiaca fruit (MPFE) was (250 and 500mg/kg, p.o.) evaluated for locomotor activity in mice. The 14 days treatment of MPFE (250 and 500mg/kg,p.o.) was investigated in the forced swim test (FST) and tail suspension test (TST). The effect of Haloperidol (0.1mg/kg, i.p., dopamine antagonist) and Bromocriptine mesylate (2mg/kg, i.p., dopamine agonist) on the antidepressant like action of MPFE were also studied in all the tests. Further, the level of brain neurotransmitters (norepinephrine, dopamine and serotonin) was assessed after 14 days treatment of MPFEin mice. 7 days and 14 days treatment of MPFE produced significant reduction of the immobility time in FST and TST respectively. Antidepressant potential of MPFE was reduced by Haloperidol (0.1mg/kg, i.p.) and increased by Bromocriptine mesylate (2mg/kg, i.p.). The neuro chemical estimation revealed the level of norepinephrine, dopamine and serotonin were increased with 14 days MPFE treatment. The behavioral and biochemical results of the present study indicate antidepressant property of MPFE, which may be mediated by the monoaminergic system in the mice.
Keywords: Musa paradisiaca (Linn.), antidepressant, dopamine, norepinephrine, serotonin, forced swim test, tail suspension test
According to WHO report (2001), approximately 450 million people suffer from a mental or behavioral disorder.1 Many medicinal plants have been used to treat such conditions. Musa paradisiaca (Linn) commonly known as plantain banana (family Musaceae) is a world popular fruit growing in tropical and subtropical regions indigenous to India and Burma. Banana is important in many parts of the world, due to its high nutritional properties. All parts of Musa paradisiaca possess valuable medicinal properties and there is a heavy demand of it in Indian as well as International markets. Plant is traditionally reported as nervine tonic. Besides its traditional use for the treatment of diarrhoea, dysentery, intestinal lesions in ulcerative colitis, diabetes, spur, uraemia, nephritis, gout, hypertension and cardiac disease,2 it has many therapeutic value in the different parts of the plant. Plant is reported for anti-diarrhoeal activity, anti-ulcerative activity, anti-microbial activity, hypoglycemic activity, antioxidant activity, anti-hypertensive activity, antiatherosclerotic activity, anti-malarial activity, anti-snake venom activity, mutagenicity, hepatoprotective activity, hair growth promoting activity and analgesic activity.3‒5
Different phytochemicals have been present in various parts of the plant which include carbohydrate, proteins (albumin and globulin, glutelin, prolamines and proteoses), free amino acids (arginine, asparagines, aspartic acid, glutamine, serine, leucines and threonine), essential amino acids (tryptophan, isoleucine, arginine), minerals (calcium, iron, potassium, sodium, phosphorus), vitamins (thiamine, niacin, pyridoxine, biotin, inositol, folic acid, tocopherols), catecholamines (norepinephrine, serotonin, dopamine), flavanoids and related compounds (Leucocyanidin, quercetin and its 3-O-galactoside, 3-O-glucoside, and 3-O-rhamnosyl glucoside) and acyl steryl glycosides (sitoindoside-I, sitoindoside-II, sitoindoside-III, sitoindoside-IV) and steryl glycosides (sitosterol gentiobioside, sitosterol myo-inosityl–beta-D-glucoside).3
It is reported that banana contains high concentration of serotonin, norepinephrine and dopamine in the fruit.6 Pulp of fruit contain serotonin (8-50μg/g), norepinephrine (1.9μg/g), dopamine (7.9μg/g) while peel of fruit contain serotonin (47-93μg/g), norepinephrine (122μg/g), dopamine (700μg/g).6 Banana also contains tryptophan that helps in restoration of essential neurotransmitters. Also, banana is a rich source of various nutritional elements that can help in treatment of mood disorders. Depression is a disorder characterized by a broad range of symptoms, including altered mood and cognitive functions, and recurrent thoughts of death or suicide.7 The World Health Organization (WHO) predicts that depression will become the second leading cause of premature death or disability worldwide by the year 2020.8 The monoamine hypothesis of depression states that it is caused by functional deficit of monoamines (norepinephrine, serotonin and dopamine) at certain sites in the brain.9 In the light of above report and recent findings, the present study was designed to investigate monoaminergic system mediated antidepressant action of Musa paradisiaca using various experimental models.
Plant material and preparation of the hydroalcoholic extract of seeds of M. pruriens (MPE)
Fruits of Musa paradisiaca Linn were procured from the local market and were identified and authenticated by Dr. DB Patel, Professor & Head, Department of Botany, Anand Agriculture University, Anand, Gujarat, India (Authentification no: AAU/BACA/GPB/615/13). The unripe fruits of Musa paradisiaca Linn were dried in shade and ground to get a coarse powder to mesh size 40. The powdered plant material was extracted exhaustively with 50% ethanol using soxhlet apparatus to obtain the hydroalcoholic extract. Crude (hydroalcoholic) extract was filtered and dried under reduced pressure at 40°C (yield-12.3% w/w of dried powdered material). Freshly prepared aqueous solution of the dried extract (MPFE) in doses of 250mg/kg, and 500mg/kg were used for pharmacological study.
Preliminary phytochemical screening
The qualitative chemical investigation of hydroalcoholic extract was carried out to check the presence of various phytoconstituents.10
Animals
Swiss mice (20-25g) of either sex (total 150) bred in Central Animal House facility of the institute were used. These animals were housed under standard conditions, maintained on a 12h light/dark cycle and had free access to food and water up to the time of experimentation. The animals were acclimatized to the laboratory environment 1h before the experiments. Animals were randomly distributed into groups of 6 animals each. All experiments were conducted during the light period (08.00-16.00h). The protocol (No: CPCSEA/IAEC/ARCP/13-14/02) of the study was approved by the Institutional Animal Ethical Committee (IAEC) and experiments were conducted according to the guidelines of CPCSEA (committee for the purpose of control and supervision of experiment on animals).
Drugs
Imipramine hydrochloride (standard drug) was obtained as gratis sample from Psychotropic India Ltd., Faridabad. Haloperidol (Seranace®, RPG Life science, India) was used as dopamine receptor (D2) antagonist. Bromocriptine mesylate (Solvay Pharma India Limited) was used as dopamine receptor (D2) agonist. Haloperidol was diluted in distilled water which was used for a vehicle of injection. Bromocriptine mesylate was dissolved in one drop of glacial acetic acid and made up to volume in distilled water. Imipramine was dissolved in 0.9% normal saline.
Forced swimming test (FST)
Mice were divided in to ten groups, each group consisting of six animals. Groupings and treatment protocol are shown in Table 1. One hour after MPFE and Imipramine or 30min after Haloperidol and Bromocriptine administration, each animal was subjected to FST on 7th and 14th day of treatments. Mice were made to swim individually in a polypropylene vessel (30×15×30cm) with a water level of 15cm at 25±2°C. Two swim sessions were conducted, an initial 15min pretest followed by a 5min test 24h later. The duration of immobility, characterized by complete cessation of swimming with the head just floating above water level was determined during the final 5min period of test.11 A decrease in the duration of immobility was indicative of an antidepressant effect.
Groups |
Drug and dose |
I (control) |
Normal saline (10ml/kg, p.o.) for 14 days |
II (positive control) |
Imipramine (10mg/kg, p.o.) as standard for 14 days |
III (test) |
MPFE (250mg/kg, p.o.) for 14 days |
IV (test) |
MPFE (500mg/kg, p.o.) for 14 days |
V (dopamine antagonist) |
Haloperidol (0.1mg/kg, i.p.) for 14 days |
VI (test+dopamine antagonist) |
MPFE (500mg/kg, p.o.) for 14 days and haloperidol (0.1mg/kg, i.p.) 1h after the last dose of MPFE |
VII (dopamine agonist) |
Bromocriptine mesylate (2mg/kg, i.p.) for 14 days |
VIII (test+dopamine agonist) |
MPFE (500mg/kg, p.o.) for 14 days and bromocriptine (2mg/kg, i.p.) 1h after the last dose of MPFE |
Table 1 Study groups for forced swimming test and tail suspension test
Tail suspension test (TST)
TST was performed using another 8 groups of mice each consist of six animals. Groupings and treatment protocol are shown in Table 1. One hour after oral and 30min after intraperitoneal administration, each animal was submitted to TST. Mice both acoustically and visually isolated were suspended on the edge of a table 50cm above the floor by the adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was recorded during a 5min period.12 Animal was considered to be immobile when it did not show any movement of body and hung passively.
Measurement of neurotransmitters
After tail suspension test, mice were sacrificed and brains were isolated. Whole brain of each mouse was weighed without thawing and immediately homogenized in 5ml ice cold acidified butanol. The homogenate was centrifuge at 500rpm at 40C for 10min and the supernatant was collected. Norepinephrine (NE), Dopamine (DA) and serotonin (5-HT) neurotransmitters were estimated by flourimetric method of Jacobowitz and Richardson (1978).13
Norepinephrine (NE) estimation
2ml of supernatant was taken in centrifuge tube containing 1.5ml of phosphate buffer. Tube was vortexed for 20sec. NE was extracted in phosphate buffer. After centrifugation at 3000rpm, 1ml of phosphate buffer extract was taken in a test tube. Now 0.25 of ice-cold versene (4g EDTA, 10N NaOH in 100ml of distilled water) was added to the phosphate buffer extract, vortexed briefly followed by addition of 0.2ml of iodine solution (4.8g potassium iodide and 0.25g of iodine in 100ml distilled water), 0.25ml of fresh alkaline sodium sulfite (2.5g of Na2SO3 in 100ml of 4N NaOH), and 0.3ml of 5N acetic acid. The cocktail was kept in boiled water for 5min and then ice for 1min. Fluorescence for NE was measured at 385 and 485nm. All values were expressed as ng/g wet tissue.
Dopamine (DA) estimation
The assay represents a miniaturization of the trihydroxide method. To 0.02ml of HCl phase, 0.05ml 0.4M and 0.01ml EDTA/Sodium acetate buffer (pH 6.9) was added, followed by 0.01ml iodine solution (0.1M in ethanol) for oxidation. The reaction was stored after two minutes by addition of 0.01ml Na2SO3 in 5m NaOH. Acetic acid was added 1.5 minutes later. The solution was heated to 100ºC for 6 minutes. When the sample would again reach the room temperature, excitation and emission spectra were read. Fluorescence was measured at 330-375nm and the values were expressed in ng/g wet tissue.
Serotonin (5-HT) estimation
2ml of supernatant was taken in glass stoppered centrifuge tube containing 5ml of heptane and 0.5ml of 0.1N HCl. Tube was vortexed for 20sec, 5-HT was extracted in 0.1N HCl. After centrifugation at 3000rpm 0.3ml HCl extract was taken in test-tube. Now 0.2ml of orthophaldehyde (OPT) solution (50mg/100ml of absolute methanol) was added to 0.1N HCl extract, followed by addition of 1.5ml of concentrated HCl (10N). The mixture was vortexed in boiling water for 10min and cooled under tap water. Fluorescence for 5-HT was measured at 360 and 470nm. All values were expressed as ng/g wet tissue.
Locomotor activity
The locomotor activity of mice was measured using an actophotometer. The movement of the animal cuts off a beam of light falling on the photocell and a count was recorded and displayed digitally. Each mouse was placed individually in the actophotometer for 2min for acclimatization and thereafter basal locomotor activity score was expressed in terms of total counts per 5min. Mice were randomly divided into two group six mice in each group. One group was treated with MPE 100mg/kg orally for 7 days. Other group was treated with MPE 200mg/kg, orally for 7 days. Mice were individually placed again in the actophotometer after 1h and 7 days of treatment for recording the locomotor activity score.14
Statistical analysis
Results were expressed as mean(s)±SEM. The statistical significance of the difference between groups for the various treatments were determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test. P<0.05 was considered statistically significant as compared to control.
Preliminary phytochemical screening
Phytochemical screening revealed the presence of carbohydrates, proteins, amino acids, alkaloids, flavanoids, tannins and phenolic compounds in the hydroalcoholic extract of MPFE.
Forced swim test (FST)
As shown in Table 2, significant (P<0.05) and dose dependent reduction in the immobility time was observed with 7 days and 14 days MPFE (250 and 500mg/kg, p.o.) treatments as compared to control. Similarly, positive control imipramine (10mg/kg, p.o.) with 7 days and 14 days treatment also produced antidepressant action as indicated by a significant reduction in the immobility time. Results of action of haloperidol and modification of haloperidol immobility time by MPE in FST are shown in Table 2. Haloperidol (a dopamine antagonist) treatment (0.1mg/kg, i.p.) group showed a significant rise in the immobility time as compared to control. When haloperidol (0.1mg/kg, i.p.) was administered 30min after the last dose of 7 days and 14 days MPFE treatment (500mg/kg, p.o.) and subjected to FST, this dopaminergic antagonist showed significant and dose dependent reversal of anti-immobility action of MPFE (500mg/kg, p.o.) as compared to MPFE treatment alone (Table 2). 7 days and 14 days of treatment with bromocriptine (a dopamine agonist) (2mg/kg, i.p.) showed significant anti-immobility action as compared to control. When bromocriptine administered 30min after the last dose of 7 days and 14 days MPFE treatment and subjected to FST, this dopaminergic agonist produced significant potentiation of anti-immobility action of MPFE (500mg/kg, p.o.) as compared to MPFE treatment alone.
Groups |
Treatments |
Immobility period (seconds) |
Immobility period (seconds) |
I |
control |
178.87±3.65 |
174.29±1.27 |
II |
Imipramine |
119.66±20.25* |
109.54±23.14* |
III |
MPFE (250mg/kg, p.o.) |
98.54±3.44* |
89.93±2.81* |
IV |
MPFE (500mg/kg, p.o.) |
87.75±4.35* |
83.28±3.04* |
V |
Haloperidol |
232.04±4.78# |
236.08±4.46* |
VI |
MPFE+Haloperidol |
185.60±4.38## |
177.76±1.87# |
VII |
Bromocriptine |
61.53±5.92# |
55.48±2.50* |
VIII |
MPFE+Bromocriptine |
40.13±1.31## |
40.49±2.57# |
Table 2 Effect of MPFE and its modulation by haloperidol and bromocriptine mesylate in forced swim-test (FST)
Data were analysed using mean±SEM and one way analysis of variance (ANOVA) followed by Tukey’s test. *p<0.05 when compared with control. #p<0.05 when compared with MPFE (500mg/kg).)
Tail suspension test (TST)
Results of TST are shown in Table 3. There was significant (P<0.001) and dose dependent reduction in the immobility time observed with 7 days and 14 days MPFE (250 and 500mg/kg, p.o.) treatments as compared to control. Similarly, positive control 7 days and 14 days imipramine (10mg/kg, p.o.) treatment showed anti-immobility activity, which further confirmed its antidepressant action. Results of action of haloperidol and modification of haloperidol immobility time by MPFE in TST are shown in Table 3. Haloperidol (a dopamine antagonist) treatment (0.1mg/kg, i.p.) group caused a significant rise in the immobility time at 7th day and 14th day of treatment as compared to control. Haloperidol (0.1mg/kg, i.p.) administration after 30min of last dose of 7 days and 14 days MPFE treatment (500mg/kg, p.o.) caused significant and dose dependent reversal of anti-immobility action of MPFE. Bromocriptine (a dopamine agonist) 7 days and 14 days treatment (2mg/kg, i.p.) showed significant reduction of immobility time as compared to control. Bromocriptine administration after 7 days and 14 days pretreatment with MPFE (500mg/kg, p.o.) showed significant and dose dependent potentiation of anti-immobility action of MPFE as compared to MPFE treatment alone.
Groups |
Treatments |
Immobility period (seconds) |
Immobility period (seconds) |
I |
control |
179.12±6.91 |
177.59±5.18 |
II |
Imipramine |
137.93±28.43* |
134.68±27.18* |
III |
MPFE (250mg/kg, p.o.) |
119.30±15.47* |
118.97±15.63* |
IV |
MPFE (500mg/kg, p.o.) |
109.23±17.12*# |
105.37±29.02* |
V |
Haloperidol |
216.43±25.95# |
210.14±24.25* |
VI |
MPFE+Haloperidol |
180.138±17.05## |
172.20±17.90# |
VII |
Bromocriptine |
81.99±9.04# |
77.24±8.60* |
VIII |
MPFE+Bromocriptine |
75.05±15.24## |
67.69±15.61# |
Table 3 Effect of MPFE and its modulation by haloperidol and bromocriptine mesylate in tail suspension test (TST)
Data were analysed using mean±SEM and one way analysis of variance (ANOVA) followed by Tukey’s test. *p<0.05 when compared with control. #p<0.05 when compared with MPFE (500mg/kg).
Measurement of neurotransmitters
Eight treatment groups mentioned in Table 1 are indicated as G1 to G8.
Norepinephrine (NE) estimation
As shown in Figure 1, increase in brain NE level concentration with 14 days MPFE (250 and 500mg/kg, p.o.) treatments as compared to control but were not found statistically significant. Also, treatment with Imipramine (10mg/kg, p.o.) showed non-significant rise in brain NE level. Haloperidol (0.1mg/kg, i.p.) treatment did not show significant change in brain NE level as compared to control. No significant modification was observed, when Haloperidol was administered 30min after last dose of MPFE treatment. While, Bromocriptine mesylate (dopamine agonist) (2mg/kg, p.o.) treatment showed significant increase in brain NE level as compared to control. When Bromocriptine mesylate administered after last dose of MPFE treatment and brain NE level concentration was measured, this dopamine agonist produced significant potentiation of effect of MPFE on brain NE level as compared to MPFE alone.
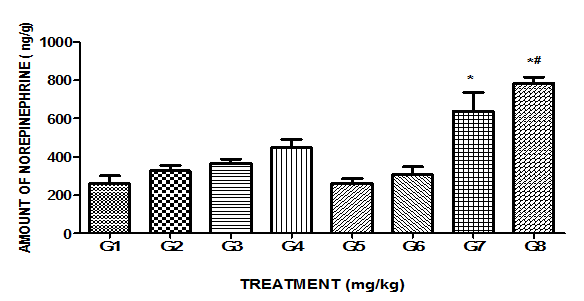
Figure 1 Effect of MPFE and its modulation by haloperidol and bromocriptine mesylate on brain norepinephrine level.
Each bar expressed as mean±SEM (n=3). Data were analyzed by one way ANOVA followed by Tukey’s test. *p<0.05 when compared with control. #p<0.05 when compared with MPFE.
Dopamine (DA) Estimation
As shown in Figure 2, non-significant rise in brain DA level concentration was observed with 14 days MPFE (250 and 500mg/kg, p.o.) treatment as compared to control. Also, treatment with Imipramine (10mg/kg, o.p.) showed non-significant rise in brain DA level. As shown in Figure 2, Haloperidol (dopamine antagonist)(0.1mg/kg, i.p.) treatment showed decrease in brain DA level concentration as compared to control but not statistically significant. When Haloperidol administered 30min after last dose of MPFE treatment and brain DA concentration was measured, this dopamine antagonist did not reduce MPFE mediated rise of brain DA level significantly as compared to MPFE alone. While, Bromocriptine mesylate (dopamine agonist)(2mg/kg, i.p.) treatment showed significant increase in brain DA level concentration as compared to control. When Bromocriptine mesylate administered after last dose of MPFE treatment and brain DA level concentration was measured. This dopamine agonist produced non-significant potentiation of effect of MPFE on brain DA level as compared to MPFE alone but significant rise in brain DA level as compared to control.
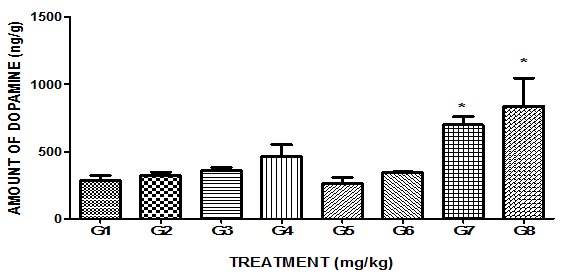
Figure 2 Effect of MPFE and its modulation by haloperidol and bromocriptine mesylate on brain dopamine level.
Each bar expressed as mean±SEM (n=3). Data were analyzed by one way ANOVA followed by Tukey’s test. *p<0.05 when compared with control.
Serotonin (5-HT) estimation
As shown in Figure 3, significant increase in brain 5-HT level concentration with 14 days MPFE (500mg/kg, p.o.) treatment as compared to control but no significant rise was observed by MPFE (250mg/kg, p.o.). Also, treatment with Imipramine (10mg/kg, p.o.) showed non-significant rise in brain 5-HT level. As shown in Figure 3, Haloperidol (dopamine antagonist)(0.1mg/kg, i.p.) treatment did not show significant change in brain 5-HT level concentration as compared to control. When Haloperidol administered 30min after last dose of MPFE treatment and brain 5-HT concentration was measured. This dopamine antagonist produced significant reversal of effect of MPFE mediated rise of brain 5-HT level as compared to MPFE alone. Bromocriptine mesylate (dopamine agonist)(2 mg/kg, i.p.) treatment did not show significant increase in brain 5-HT level concentration as compared to control. When Bromocriptine mesylate administered after last dose of MPFE treatment and brain 5-HT level concentration was measured. This dopamine agonist did not potentiate the effect of MPFE but showed significant rise in brain 5-HT level as compared to control.

Figure 3 Effect of MPFE and its modulation by haloperidol and bromocriptine mesylate on brain serotonin level.
Each bar expressed as mean±SEM (n=3). Data were analyzed by one way ANOVA followed by Tukey’s test. *p<0.05 when compared with control. #p<0.05 when compared with MPFE. ##p<0.05 when compared with MPFE.
Locomotor activity
Acute oral administration of hydroalcoholic extract of Musa paradisiaca fruit (MPFE) at the dose of 250mg/kg did not show any significant change in the locomotor activity of mice after 1hr (878±25.95) as compared to baseline control (877.66±24.62). Also, 7 days treatment of MPFE (250mg/kg) did not showed significant changes in the locomotor activity (920.5±19.53) of mice as compared to baseline control. Similarly, 14 days treatment of MPFE (250mg/kg) showed non-significant increase in the locomotor activity (938.66±18.79) of mice as compared to baseline control. Similarly, MPFE at the dose of 500mg/kg did not show any significant change in the locomotor activity of mice after 1hr (835.16±55.94) as compared to control to baseline control (834.5±57.15 ). 7 days treatment of MPFE (500mg/kg) showed slight increase in the locomotor activity (906.66±36.51) of mice as compared to baseline control. Whereas, 14 days treatment of MPFE (500mg/kg) also showed non-significant increase in the locomotor activity (940±31.19) of mice as compared to baseline control.
In the present study, 7 days and 14 days treatment of hydroalcoholic extracts of Musa paradisiaca fruits produced significant antidepressant activity in forced swim test and tail suspension test. FST and TST are widely used to screen new antidepressant drugs.11,12 Characteristic behavior scored in these tests is termed immobility, reflecting behavioral despair as seen in human depression.12,15 It has been seen that TST is less stressful and has higher pharmacological sensitivity than FST.16 According to our result, the antidepressant like effect of MPFE was significantly potentiated by bromocriptine (a dopamine D2 receptor agonist) and reversed by haloperidol (a dopamine D2 receptor antagonist) suggesting that antidepressant action of MPFE mediated via interaction with dopaminergic system. In order to exclude the possibility of MPFE exerting such a psycho-stimulant like effect, an additional locomotor activity test to check the motor stimulating activity, was performed. Further, it should be pointed out that the antidepressant-like effect of MPFE in the FST and TST do not seem to be associated with any stimulating locomotor activity, as at doses similar to that causing a marked antidepressant-like action did not affect spontaneous motor activity. MPFE at the tested dose level did not produce any behavioral changes or motor dysfunction in the locomotor activity test after either the acute or repeated treatment. This result confirms the assumption that the antidepressant like effect of the MPFE at the tested doses in the FST and TST are specific. Furthermore, the results observed with MPFE treatment were largely comparable to imipramine, which suggests that MPFE may produce a selective antidepressant effect.
In recent years, a number of lines of evidence have begun to shed light on the mechanisms of antidepressant drugs. One research work suggested that monoamine dopamine and especially the D2-like family of dopamine receptors might play a crucial role in mediating the action of antidepressant treatments.17 There is also a report that dopamine D2 receptor activation could reduce immobility time.18 In the present study, the obtained results suggest the involvement of dopamine D2 receptor agonistic nature of MPFE in mediating its antidepressant action. Intensive research into the neurobiology of depression suggests that an increase in the level of monoamines at the synapse is believed to be the first step in a complex cascade of events that ultimately results in antidepressant activity. This study also provided neuro chemical evidence for the involvement of particular monoamines including dopamine, serotonin and norepinephrine in the antidepressant like effects of MPFE.19
The main biochemical theory of depression is the monoamine hypothesis which predicts that the major neuro chemical process in depression is the impairment of monoaminergic functions associated with decreased levels of serotonin, norepinephrine and dopamine. Effective antidepressant treatments normalize the disturbed monoaminergic systems which are assumed to be responsible for the clinical features of depression.20 In the present study, measurement of mice brain monoamine neurotransmitters such as dopamine, norepinephrine and serotonin after 14 days MPFE treatment (250 and 500mg/kg, p.o.) and their modification with Haloperidol (0.1mg/kg, i.p.) and Bromocriptine mesylate (2mg/kg, i.p.) were performed. The result showed that significant rise in brain serotonin level was observed with MPFE (500mg/kg, p.o.) while, non-significant rise in dopamine and norepinephrine levels were observed. These indicated that antidepressant activity of MPFE may be mediated by dopaminergic &/or noradrenergic &/or serotonergic mechanisms. Bromocriptine (dopamine agonist) (2mg/kg, p.o.) significantly increased in brain NE and DA level and significant potentiation in NE and DA level of MPFE observed by Bromocriptine treatment. Haloperidol (dopamine antagonist) (0.1mg/kg, p.o.) did not show significant change in brain NE, dopamine and 5-HT level. Also, Haloperidol did not modified effect of MPFE on brain NE and DA level. But significant reversal of MPFE mediated rise on brain 5-HT was observed with Haloperidol treatment. Imipramine is a well-known antidepressant agent. It showed significant antidepressant activity in behavioral models but did not show significant change in brain NE, DA and 5-HT level.
The efficacy of most herbal remedies is attributed to various active principles in combination. Results of phytochemical screening showed presence of alkaloid, carbohydrates, tannins, amino acids, flavanoids and proteins in the fruits. High concentration of serotonin, norepinephrine and dopamine and tryptophan in the fruit may help in restoration of neurotransmitters responsible for observed antidepressant activity.6 Reported study also provided support for nervous activity of Musa paradisiaca fruits on monoaminergic systems.21 In conclusion, the present study provides scientific evidence that the antidepressant action of Musa paradisiaca fruits is mediated via interactions with the monoaminergic systems. Future work is directed for identification and isolation of active constituents responsible for antidepressant activity.
Facilities used for research work was provided from AR College of Pharmacy & GH Patel Institute of Pharmacy, Vallabh Vidyanagar-388120.
The author declares that there is no conflict of interest.

©2018 Darji, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.