eISSN: 2379-6367


Research Article Volume 5 Issue 2
School of Health Sciences, Department of Pharmacy, Division of Pharmaceutical Technology, National and Kapodistrian University of Athens, Greece
Correspondence: Marilena Vlachou, Faculty of Pharmacy, Department of Pharmaceutical Technology, National and Kapodistrian University of Athens, Greece, Tel +302107274674, Fax 3.02E+11
Received: February 02, 2017 | Published: March 30, 2017
Citation: Vlachou M, Siamidi A, Efentakis M. Investigation of a novel “tablets in capsule” theophylline formulation system for modified release. Pharm Pharmacol Int J. 2017;5(2):51-56. DOI: 10.15406/ppij.2017.05.00115
In pharmaceutical research, new dosage forms that modify drug release are useful for circadian rhythm related diseases. The aim of this investigation is to examine the release behavior of multiple-unit modified-release formulations (‘tablets in capsule’) of theophylline. Mono-layered and three-layered minitablets, filled into capsules, were prepared using theophylline and dextran or pectin, as excipients. Their release behavior was compared with respect to powder filled capsules and the commercially available Theodur® 20mg tablets. Dissolution tests were performed in three pH media (1.5, 7.4 and 6.0) in the presence and absence of the enzyme Pectinex® Ultra SP-L solution, which degrades polysaccharides. The results indicate that enteric-coated capsules showed no drug release, in the first two hours in the acidic media. Dextran produces a thicker gelled layer than pectin and therefore, dextran based 'tablets in capsules' systems are suitable as a model extended release formulation. The drug is released from 3-layered minitablets at a slower rate compared to matrix minitablets. The product, Pectinex® Ultra SP-L, was found to give more accurate dissolution results when used as an additive to the media, mimicking the large intestine/colon area especially when the formulations target this region.
Keywords: theophylline, modified release, tablets-in-capsule, dextran, pectin, pectinex® ultra spl, tablets-in-capsule
COPD, chronic obstructive pulmonary disease; SUDFs, single unit dosage forms; MUDFs, multiple unit dosage forms; MDT, mean dissolution time; D.E.%, dissolution efficiency
Theophylline is a methylxanthine drug used in therapy for respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma. A chronotherapeutic approach towards asthma management is particularly important, as bronchial asthma attacks follow circadian rhythms, since they appear more often during the night and/or early in the morning. Many chronotherapeutic studies have shown the benefits of antiasthmatic medication when they are taken at nighttime and the drug is released in specified times, according to the disease pathophysiology, in order to increase the therapeutic effect and help towards patience compliance.1 Therefore, theophylline was chosen in the present study as a model active pharmaceutical ingredient as the time of drug release after administration requires a modified release formulation. Lopes and his colleagues (2006) demonstrated that the per os administration of controlled release formulations can be classified into two broad categories: Single unit dosage forms, (SUDFs), like tablets and capsules, and multiple unit dosage forms (MUDFs), like granules, pellets or minitablets. MUDFs offer a high control of drug release, because the dose is administered in many single units and is the sum of the drug in each subunit. Advantages of MUDFs include: smaller inter- and intra-variability, less risk of dose dumping, higher degree of dispersion in the gastrointestinal system, lessening the risk of high local concentrations.2 Minitablets offer an alternative dosage form for pellets having low porosity, same shape and size, smooth surfaces with small batch-to-batch variability and are easy to produce.3 On the other hand, formulating MUDFs with minitablets can become a complex and expensive procedure, since the capsule filling is a tedious process and the minitablet production demands extra care and fine adjustments of the instruments used.4 Additionally, multilayer tablets are usually composed of two, three or more layers, consisting of the same or different substances and/or excipients. The most important reason of multilayer tablet production is the control over the active substance release. Initially, the burst effect can be avoided and the drug's dissolution and release can be effected by using various polymers; thus various systems for extended, modified, quick/slow, biphasic, or pulsatile release, have been investigated.5 Dextran and pectin are polymeric excipients, which when in contact with water molecules absorb them, swell and produce a gel layer. This formation can act as a barrier, keeping the active substance entrapped in the system for a period of time allowing control over the drug release profile. Multilayer tableting is a low cost and effective system for a variety of active substances and excipients and can be integrated in minitableting with analogous results, regarding control release. According to the literature, in the large intestine there is a variety of bacteria species producing a plethora of hydrolytic and reductive enzymes, while performing various processes, such as hydrocarbon and protein fermentation. The major energy source, for such anaerobic bacteria, is hydrocarbons, e.g. non-amyloid polysaccharides. In order to mimic the colon micro-environment, while studying drug release rates in the large intestine, buffer solutions containing enzymes that degrade polysaccharides are used. An ideal commercial product to mimic the intestinal micro-environment is Pectinex® Ultra SPL, which is an enzymatic dispersion obtained from the microorganism Aspergillus aculeatus, exhibiting β-galactosidase activity. Many researchers have used Pectinex® Ultra SP-L, when performing dissolution tests in various concentrations. Fernandez-Hervas and Fell (1998) performed dissolution tests in a concentration of about 44200PG/L, while6 in a concentration of about 86600PG/L and7,8 in a concentration of about 61800PG/L. The aim of the present research is to design, prepare and evaluate solid pharmaceutical multi-unit dosage forms of theophylline administered orally, for targeted release at the intestine area, using simple and enteric coated capsules filled with minitablets, composed of either layers or mixtures of the active ingredient and the polymeric excipients dextran and pectin. These dosage forms were chosen in order to delay the onset of the drug release for a period of time equal to the transit time through the stomach (lag time ≥2hours) and then gradually release the active substance. These new formulations were also tested in reference to powder filled capsules/enteric coated capsules and versus the commercially available formulation Theodur® 200mg tablets. The dissolution tests were carried out in three pH media in the absence and presence of the enzyme dispersion of Pectinex® Ultra SP-L.
Materials
The following chemicals were purchased from Sigma-Aldrich Chemie GmbH, Steinheim, Germany and used as received: Theophylline (anhydrous, minimum 99%), Dextran (from Leuconostoc mesenteroides, average molecular weight 5x106-40x106), Pectin (pectin from citrus fruits). Theodur®, 200mg prolonged release tablets (Lavipharm Hellas, Athens, Greece), was purchased from a local pharmacy store. Pectinex® Ultra SP-L (3800 PGNU/ml, Novozymes A/S, Bagsvaerd DK-2880 DENMARK 8890168 Polygalacturonase), a gift from Novo Nordisk Hellas Ltd, Athens, Greece, was used as an additive into the buffer solution during dissolution testing.
Methods
‘Tablets in capsule’ device preparation: In the present study, flat minitablets 6.5 mm in diameter, were compressed to a hardness of 8-10kp on a hydraulic press (Carver Inc. model 3393).2,9,10 Two different mini-tablets were prepared: a. minitablets consisting of 3 layers, where the 2 outside layers where formed from the excipient (2 x 25mg of either pectin or dextran) and the middle layer, which was formed from the active drug substance (50mg of theophylline) (Figure 1A) (Figure 1B). Minitablets consisting of a mixture of the active drug substance (50mg of theophylline) and the excipient (50mg of either pectin or dextran) (Figure 1B). After preparation, 4 minitablets of same consistency were used to fill the size zero simple and enteric-coated capsules.
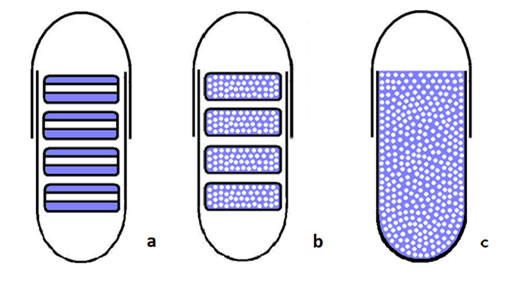
Powder-filled capsules: Capsules were filled with a mixture of the drug substance and excipients, each one containing 200mg of Theophylline and 200mg of the excipient (either pectin or dextran) (Figure 1C).
In vitro drug release studies: The drug release from each capsule was carried out in a USP dissolution paddle (PharmaTest-D17) at 100 rpm and 37±0.5οC. All dissolution studies were performed in triplicate. Buffer solutions were used as dissolution media to simulate the conditions in the gastrointestinal tract. For the first 2h, 400ml of an HCl solution (0.03 M), 0.2 w/v NaOH, pH 1.5 was used (Buffer solution A) to simulate the conditions in the stomach. For the next 3h, 400ml of a K2HPO4 solution (0.2 M), pH 9.4, was added to the buffer solution A to produce buffer solution B, pH 7.4, in order to simulate the conditions in the small intestine. For the last 7h, 200ml of a H3PO4 solution (0.17 M), pΗ 1.5, was added into the vessel to produce buffer solution C, pΗ 6.0, to simulate the conditions in the large intestine. Last, in order to test the effect of Pectinex® Ultra SP-L, the above experiments were repeated with the addition of 12ml of Pectinex® Ultra SPL 3800 PGU/ml in the final solution. Samples (5ml) were withdrawn at predetermined time intervals (every 30min for the first 120min and every 60min thereafter), filtered and analyzed using a Perkin–Elmer UV spectrophotometer (Norwalk, CT) at λmax =273.
Comparison of the dissolution profiles: In order to compare the dissolution profiles, graphs of % drug release (mean±standard deviation) vs time were produced and t20%, t50% t90%, and the values of Mean Dissolution Time (MDT) and Dissolution Efficiency (D.E.%) were calculated. The values of t20%, t50% and t90% relate to the time, where the 20%, 50% and 90% of the drug is released. MDT is the value used to characterize the drug release rate from a dosage form and in actual practice, the following equation 1 is used to derive an estimate of MDT from experimental dissolution data:11
Eq. 1
Where, W∞ is the asymptote of the dissolved amount of drug and ABC is the area between the cumulative dissolution curve and W∞.
According to Khan (1975), D.E. (%) is a parameter useful for the evaluation of dissolution in vitro and is calculated according to equation 2:
Eq. 2
Where, y is the percentage of dissolved product and D.E. the area under the dissolution curve between time point’s t1 and t2, expressed as a percentage of the curve at maximum dissolution, y100 over the same time period. When a relationship between dissolution and another variable is sought, is considered more realistic to use D.E. (%), which takes into account the dissolution profile as a whole. In addition, where a quantitative comparison is required, D.E. (%) is a more suitable parameter and when limits are set on D.E. (%) it can be used for quality control in place of the conventional dissolution level.12
Statistical analysis: Results were expressed as the mean ± standard deviation (SD) and analyzed using unpaired t-test (P<0.05 for significantly different results).
Comparison between simple capsules and enteric-coated capsules
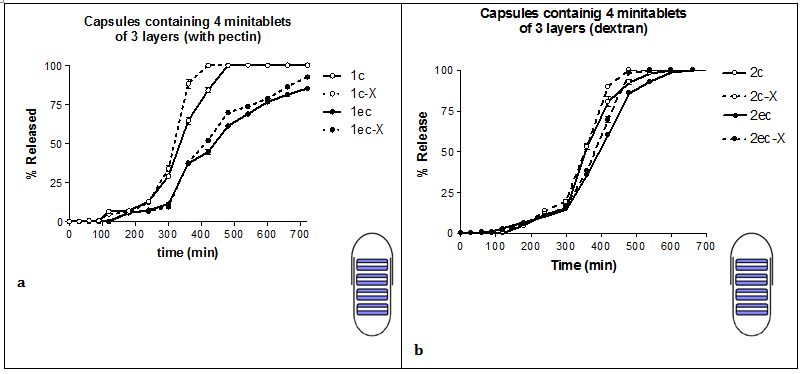
Drug delivery researches are concerned with the development of effective and selective drug delivery systems, able to release the active ingredients in a modified release rate. This goal can be achieved through enteric coating of the prepared dosage form.13 In our study, enteric-coated capsules played an important role in the initial drug release retardation. In all cases during the first two hours (acidic pH) the release of the drug from enteric-coated capsules was 0%. Enteric coating is mostly used to protect the drug substance(s) from degradation by the acidic gastric contents, to improve their tolerability when stomach irritation occurs, to modify their release and achieving targeted release.14 Although mostly applied to tablets, can also be used to coat other solid formulations including capsules.15 Previous studies with theophylline formulations, developed systems that were able to suitably retard the onset of drug release, thus providing a colon-specific delivery (Mura et al., 2003). On the other hand, simple capsules dissolved as soon as they were in contact with the dissolution media releasing immediately their contents (Figure 2, Table 1).
Formulation No# |
Capsule Type |
Excipient |
Type of Formulation |
Pectinex® Ultra Sp-L |
Experiment |
T20% |
T50% |
T90% |
Mdt |
Mean D.E. (%) |
1 |
c |
Pectin |
Minitablets-3 layers |
- |
1c |
280 |
335 |
460 |
358,33 |
54,40 |
√ |
1c-X |
280 |
320 |
400 |
333,42 |
57,86 |
||||
ec |
- |
1ec |
320 |
430 |
>720 |
412,86 |
36,36 |
|||
√ |
1ec-X |
320 |
420 |
715 |
417,69 |
38,85 |
||||
2 |
c |
Dextran |
- |
2c |
310 |
370 |
510 |
414,35 |
46,62 |
|
√ |
2c-X |
310 |
360 |
450 |
395,06 |
49,30 |
||||
ec |
- |
2e |
320 |
380 |
475 |
385,85 |
50,58 |
|||
√ |
2ec-X |
320 |
355 |
420 |
370,75 |
52,67 |
||||
3 |
c |
Pectin |
Minitablets-mixture
|
- |
3c |
135 |
210 |
410 |
269,10 |
66,79 |
√ |
3c-X |
135 |
210 |
350 |
249,96 |
69,47 |
||||
ec |
- |
3ec |
160 |
220 |
490 |
306,38 |
61,61 |
|||
√ |
3ec-X |
160 |
220 |
350 |
270,34 |
66,62 |
||||
4 |
c |
Dextran |
- |
4c |
235 |
340 |
470 |
359,56 |
54,23 |
|
√ |
4c-X |
235 |
310 |
415 |
325,50 |
58,96 |
||||
ec |
- |
4ec |
270 |
340 |
520 |
363,98 |
49,44 |
|||
√ |
4ec-X |
270 |
320 |
415 |
341,31 |
56,76 |
||||
5 |
c |
Pectin |
Powders mixture |
- |
5c |
90 |
150 |
340 |
221,65 |
73,38 |
ec |
- |
5ec |
150 |
210 |
350 |
259,17 |
68,17 |
|||
6 |
c |
Dextran |
- |
6c |
90 |
190 |
350 |
225,37 |
72,86 |
|
ec |
- |
6ec |
150 |
210 |
360 |
265,19 |
67,33 |
|||
Theodur® 200 mg tablets |
- |
Theodur® |
135 |
380 |
>720 |
313,74 |
44,07 |
|||
√ |
Theodur®-X |
135 |
370 |
>720 |
315,33 |
47,28 |
||||
Table 1 Types of theophylline formulation used, the experimental conditions during dissolution (presence of absence of Pectinex® Ultra SP-L solution), time at 20%, 50% and 90% of drug release (t20%, t50% and t90%), the Mean Dissolution Time (MDT) and the Dissolution Efficiency (D.E.%)
Comparison between the excipients dextran and pectin
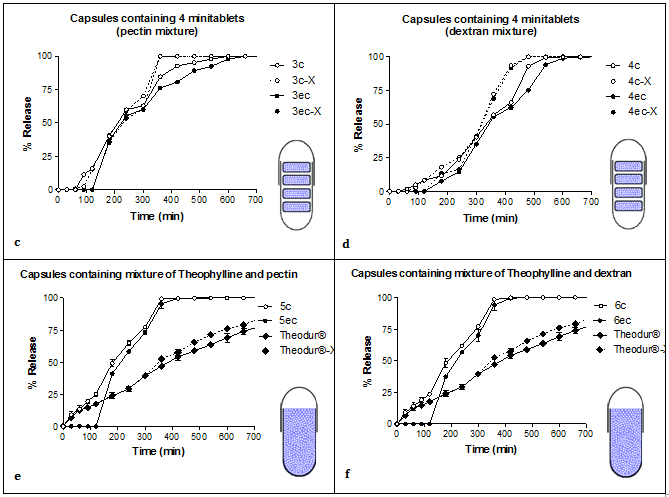
Dextran offered better extended release characteristics to the formulations when compared to pectin (comparison of the left side and the right side of (Figure 2). The values of t20%, t50% and t90% were higher when dextran was used (Table 1), showing a delay in drug release. Dextran's gelled layer is thicker than pectin's, which is less compact, allowing thus for facile water diffusion, and as a result, hydration and dissolution becomes faster (optical observation during experimental procedure). Both dextran and pectin are polymers, which upon hydration, swell and produce a gelled layer that acts as a barrier to drug release. Used tramadol hydrochloride and dextran to produce tablets and their results indicated that dextran were used polymeric chains showed fast hydration kinetics with high amount of water absorbed, which turned into fast dissolution or fast swelling as a function of the molecular weight of dextran. Many researchers have studied the use of pectin as an excipient in modified release formulations targeting the colon area. When pectin was used as a coating, prolonged release was observed, but under the influence of the enzymes, degradation of the tablet was found to be faster.6,16,17 Pectin is also a biodegradable polysaccharide, and has been used as a thickening and a gelling agent. It also has bioadhesive properties toward other gastrointestinal tissues, which can be used as a drug delivery device on a specific site for targeted release and optimal drug delivery due to intimacy and duration of contact.18
Comparison between 'tablets in capsule' system and powder filled capsules
'Tablets in capsule' systems exhibited different release rates than the powder filled capsules. In all cases the compression of powders to minitablets played an important role in reducing the release rate, t90% and MDT, in comparison to powder filled capsules (Figures 2A‒D) (Figure 2E‒F) (Table 1). 'Tablets in capsule' systems are easily produced, inexpensive formulations with promising results in modified drug release. During the past few years there is increasing interest on 'tablets in capsule' system formulations with various drug substances19 have tested, in vitro, 'tablets in capsule' system formulations containing clopidogrel and excipients in various combinations. Other researchers have tested the controlled release of mesalazine from 'tablets in capsule' systems for the targeted release of the drug in the colon area.9,20,21 'Tablets in capsule' system formulations have also been tested for biphasic release of one drug substance,20,22,23 or more.24,25 Also, in the study of26 'tablets in capsule' systems were tested in vivo for their ability to float in the stomach for more than 4h.
Comparison between 3-layered and matrix minitablets in capsule
The dissolution test for the prepared dosage forms, 3-layered and matrix minitablets in capsule were performed with the same operation and under the same testing conditions, in order to study the drug release profile and determine which formulation offers the best drug release behavior (Figure 2). The drug release was retarded in all cases when 3-layered minitablets were used in comparison to matrix minitablets. For example, the values of t20%, t50% and t90%, for formulation 1c, containing 3-layered minitablets, are 280, 335 and 460min and for formulation 3c, which contain matrix minitablets are lower; 135, 210 and 410min, respectively (Table 1). This is probably due to the fact the outside layers of the minitablets are solely composed of the polymer, which by hydration, produces a gelled layer that retards the release of the drug. Many researches have demonstrated the usefulness of multi-layered tablets.27,28 Considering various formulation parameters it is possible to get the appropriate release kinetics.29
Use of Pectinex® Ultra SP-L as an additive to the dissolution media
In all cases the presence of Pectinex® Ultra SP-L into the buffer solution, after the initial 300min, affected the release of the drug in the in-house produced formulations. The difference between the above methods is probably statistically significant (comparison of D.E. (%) values: P<0.05, Table 2). The Pectinex® Ultra SP-L solution causes the cleavage of the polymeric bonds, leading to a quicker drug release. The release of theophylline from Theodur® 200mg tablets was not affected by the presence of the Pectinex® Ultra SP-L solution. This is due to the fact that Theodur® 200mg tablets contain the excipient Hydroxy Propyl Methyl Cellulose (HPMC), which is not affected by the pectinolytic enzymes, as indicated previously in the literature.30 The formulations 5c, 5ec, 6c, 6ec were not tested with the Pectinex® Ultra SP-L solution, as the dissolution was completed in 360min.
Experimental Code |
P Value |
1c-1cX |
P < 0.001 |
1ec-1ecX |
P < 0.01 |
2c-2cX |
P < 0.01 |
2ec-2ecX |
P < 0.05 |
3c-3cX |
P < 0.01 |
3ec-3ecX |
P < 0.001 |
4c-4cX |
P < 0.001 |
4ec-4ecX |
P < 0.001 |
Theodur® -Theodur®-X |
P > 0.05 |
Table 2 Experimental codes and P values (t-test) during Dissolution Efficiency comparison for the paired formulations (presence or absence of Pectinex® Ultra SP-L solution)
The data obtained show that dextran based 'tablets in capsules' systems can be used as a model for extended release formulations. The release of the drug from 3-layered minitablets is slower in comparison to matrix minitablets. The Pectinex® Ultra SP-L product gives more accurate dissolution results, when used as an additive to the media, which mimic the large intestine/colon area, especially when the formulations to be tested target this region.
None.
Author declares that there is no conflict of interest.

©2017 Vlachou, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.