eISSN: 2379-6367


Research Article Volume 11 Issue 3
1Hospital Universitari Dexeus - Paidodex, Pediatrician, Spain
2Hospital Universitari Dexeus - Paidodex, Dermatologist, Spain
3Medichem, Pharmaceutical Development Scientist, Spain
Correspondence: Isabel Fernández, Medichem Pharmaceutical Development Scientist, Barcelona, Catalonia, Spain
Received: August 07, 2023 | Published: September 1, 2023
Citation: Gairí JM, Castany A, Fernández I. Improved barrier function and skin acceptability of a new cosmetic (body lotion) for atopic-prone skin containing octenidine and silver citrate as antimicrobial agents. Pharm Pharmacol Int J. 2023;11(3):110-116. DOI: 10.15406/ppij.2023.11.00409
Introduction: Atopic dermatitis (AD) is the most common chronic inflammatory skin disease, and affects both children and adults. The pathophysiology of atopic dermatitis is complex and involves disruption of skin barrier function, immune dysregulation, genetic susceptibility and interaction with environmental factors. Emollients are considered to be the essential basic component of any treatment, as they are effective in preventing flare-ups and in reducing the symptoms of AD.
Objective: To evaluate the skin acceptability and the effect of a new body lotion for atopic skin containing octenidine dihydrochloride and silver citrate upon skin barrier function and.
Methods: In-use test under dermatological and pediatric control, applied under normal conditions of use. Nineteen adults between 22-70 years of age (95% women) and 20 children between 7 months and 16 years of age (45% girls) applied the investigational product during four weeks. The frequency of adverse events was recorded and cosmetic acceptability and satisfaction were assessed by means of a survey in both groups, Additionally, transepidermal water loss (TEWL) was measured in the adult group.
Results: A significant decrease in TEWL versus baseline was observed after four weeks of repeated applications (10.96 ± 0.99 vs. 7.87 ± 0.41, respectively; p <0.001). There were no side effects or discomfort secondary to application of the emollient, such as irritation or discomfort, as recorded by the investigating dermatologist. The participants had a favorable opinion, particularly regarding the texture, applicability and efficacy of the product. Among the regular users of this type of product, 100% felt it to be "as good as" or "better" than the generally used product.
Conclusion: The results obtained confirm the good skin acceptability of the new emollient containing octenidine and silver citrate and suggest that may help improve skin barrier function and contribute to the relief and control of atopic dermatitis.
Keywords: atopic dermatitis, emollient, octenidine, silver citrate, skin barrier
AD, atopic dermatitis; FLG, filaggrin; MRSA, methicillinresistant S. aureus; S. aureus, Staphylococcus aureus; SC, stratum corneum; TEWL, transepidermal water loss
Atopic dermatitis (AD) is a recurrent, chronic pruritic inflammatory skin disease characterized by dysfunction of the skin barrier, sensitization to allergens and repeated skin infections that typically progress to produce flare-ups.1 It is the most common chronic inflammatory skin disorder,2 with a broad range of clinical manifestations and fluctuations in severity. Although it is most commonly observed in children (up to 20% of all children and adolescents are estimated to suffer AD), it also affects many adults (up to 10%).3 In fact, the evidence suggests that the prevalence of persistent and new cases of AD in adulthood is increasing.4 The consequences of having a skin disease may extend beyond impaired quality of life caused by discomfort (such as itching) and the presence of skin lesions.5 Atopic dermatitis can have a profoundly negative impact upon the psychological well-being of the patients and related persons.6 Previous studies have evidenced a higher prevalence of depression and anxiety in both children and adults as compared to the healthy population.7–9
The exact etiology of AD, which is a complex and multifactorial disorder, has not been precisely established, but is thought to involve the interaction of altered skin barrier function, microbiome disruption, immune dysregulation and genetic susceptibility, leading to alteration of the structural proteins of the skin barrier, which together with environmental factors (allergens, stress, etc.) trigger immune-mediated inflammation.2,10–12 One of the major pathophysiological factors in AD is dysfunction of the natural skin barrier.13 In this respect, one of the main functions of the stratum corneum (SC) or horny layer of the skin is to act as a permeable barrier preventing water loss through transcutaneous evaporation and providing an antimicrobial barrier, as well as promoting colonization by non-pathogenic bacterial flora.14 An important factor in the differentiation and growth of a normal SC is filaggrin (FLG), a structural protein essential for proper formation and function of the skin barrier. Decreased expression of FLG, and consequently of F-type keratohyalin granules, may result in a shortage of these granules, which in turn causes the stratum granulosum or granular layer to rupture, thereby affecting the SC.15–17
An equally important barrier altered in AD is the antimicrobial barrier. The antimicrobial effect of the epidermal barrier and the physical characteristics of the barrier are directly related. Therefore, rupture of the physical barrier causes water loss and facilitates the penetration of allergens. This commonly leads to colonization of the skin by Staphylococcus aureus (S. aureus). This bacterium plays an important role in the natural course of atopic dermatitis. It has long been known that S. aureus intensely colonizes the skin of patients with atopic dermatitis, and this colonization contributes to the worsening of skin inflammation18 secondary to the increased production of IgE, especially IgE targeting S. aureus toxins.19,20 However, there is no standard intervention to reduce the burden of S. aureus in AD.21
AD has traditionally been treated with emollients, topical and systemic corticosteroids, oral antihistamines, immunosuppressive agents and other therapies.21 While these agents improve the signs and symptoms of AD, studies have reported that their prolonged use can result in side effects, including but not limited to red and burned skin, skin atrophy, hypertrichosis, telangiectasias or immunosuppression in the case of the immunosuppressors that are sometimes used in moderate to severe forms of the disease, among others.22–24 Therefore, complementary therapies consisting of emollients are increasingly developed, based on the benefits of plant extracts for treatment of the disease. Moisturizing creams help reduce the signs, symptoms and inflammation of AD, reducing its severity and prolonging the interval between flare-ups of the disease. In effect, moisturizing creams are extremely useful in patients with AD and typically contain a humectant or wetting agent (which promotes stratum corneum hydration, such as urea or glycerol) and an occlusive agent (which reduces evaporation).25 Moisturizing creams minimize transepidermal water loss (TEWL) and improve stratum corneum hydration. They form an integral part of almost all AD management plans and are strongly recommended as topical treatments for AD in adults in the new clinical guide of the American Academy of Dermatology.26
A wide variety of moisturizing creams are currently available, including emollients, occlusive agents or humectants. The investigational cosmetic product is a new body lotion for the care of atopic skin and atopic-prone skin that contains emollients (glycerine, caprylic/capric triglyceride, squalene, coco-caprylate/caprate, diethyl succinate, cetearyl alcohol, bis-diglyceryl polyacyladipate-2) and active ingredients (octenidine, silver citrate, polidocanol and chamomile extract). The hydrophilic emollients (glycerine) that afford an immediate moisturizing effect, while other more lipid components (such as caprylic/capric triglyceride and squalene) that act more in depth, creating a film on the skin and between the corneocytes that prevents water from evaporating from the skin.. Octenidine dihydrochloride is a cationic surfactant with activity against gram-positive and gram-negative bacteria, including methicillin-resistant S. aureus (MRSA),27–29 that can be used for prophylactic and therapeutic purposes.30 It has also been shown to be more potent "in vitro" than chlorhexidine against some pathogens, including S. aureus, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis and Pseudomonas aeruginosa.31–33 The antimicrobial activity of silver depends on the bioavailability of the silver ion (Ag+).34,35 The high bioavailability of ions in the silver citrate complex makes it rapidly effective against a broad spectrum of bacteria, viruses and fungal species.36 Silver also has potent immunomodulatory characteristics.37 Polidocanol possesses local anesthetic properties, and is used as an antipruritic agent in galenic formulations and cosmetic products for the treatment of skin conditions associated with itching, such as eczema.38 Chamomile (Matricaria recutita L.), belonging to the Asteraceae family, contains three main sesquiterpene constituents (azulene, bisabolol and farnesene) with antiinflammatory, analgesic, antioxidant, mildly astringent, healing and antibacterial properties. It is therefore suitable for the treatment of skin disorders such as eczema.39,40
Two usability tests of the new investigational cosmetic product were carried out on healthy volunteers with sensitive skin and atopic predisposition. The first was performed on adult subjects under dermatological control and the second test on children and babies under pediatric control. The aim of both in-use tests was to evaluate the skin acceptability of this new emollient cosmetic product (body lotion for atopic skin, containing octenidine and silver citrate) when applied under anticipated conditions of use for four weeks in adults, children and infants with sensitive skin and an atopic predisposition. In addition, in the adult group, the effect of the cosmetic on the barrier function of the skin was evaluated.
In-use test in the adult population
The study was conducted in healthy adult (legal age) males and females. Inclusion criteria were: age 18 to 70 years, Caucasian origin, indifferent body skin type, experiencing itching regularly, having sensitive body skin, defined by a recent history of repeated skin discomfort (itching, burning, erythema with or without flushing, intolerance to topical products in the past year, etc.) and having a history of atopy. Exclusion criteria were: pregnant and/or lactating women, current use of other emollients or cosmetics in the application area in the last 14 days, subjects with acute dermatological processes, subjects taking anti-inflammatory and/or anti-allergic medication in the last 14 days, allergy or intolerance to cosmetic products or any of the components of the product under investigation, subjects with lesions or skin spots in the application area. The participants were required to apply the minimum amount necessary to cover the entire body with the investigational product at home twice daily (morning and evening), for 28 days. They were provided with the normal instructions for use established by the manufacturer (apply to clean, dry skin and massage until completely absorbed), and were instructed to clean the body skin as usual with a neutral bath gel that was supplied to them. The effect on the skin barrier was evaluated based on the TEWL values, skin acceptability established by clinical dermatological evaluation, and cosmetic acceptability and subjective satisfaction evaluated using a questionnaire.
Effect on the skin barrier from the TEWL values
The effect upon the epidermal barrier was analyzed based on TEWL on days 1 (baseline), 14 and 29. The measurements were made using the TewameterTM 300 device (Courage + Khazaka electronic). This device measures the water evaporation rate across the stratum corneum (g.m-².h-1), as well as relative humidity of the room air (%) and temperature (°C). The experimental value of electrical water loss capacity was taken to be the average of three tewameter measurements made at three different points of the delimited area (the center of an area measuring 35 cm² (7 x 5 cm), located on the left or right forearm). The skin protective effect was determined based on the mean values and standard errors (mean ± SEM) of the different parameters, at each study timepoint, in the explored areas. The Shapiro Wilk test was used to assess normality of the distribution of differences, and the Wilcoxon test (two-tailed, statistical significance: p <0.05) was applied to compare the different timepoints versus baseline. In the event of statistically significant variations, the corresponding percentage change versus baseline at each study timepoint was calculated from the mean values.
Skin acceptability
Skin acceptability was assessed from clinical examinations by dermatologists on days 1 and 29. Acceptability was analyzed by calculating the frequency of skin signs and reactions to the product. Consideration was also made of the frequency of subjective responses regarding the absence of skin irritation (agree/quite agree/quite disagree/disagree) recorded from the question “The product does not irritate your skin”, included in a questionnaire completed by the participants at the end of the study.
Cosmetic acceptability and satisfaction
Cosmetic acceptability and satisfaction of the participants with the product were assessed based on the subjective responses of the subjects to specific questions about cosmetic aspects (pleasantness, texture, smell, ease of application, stickiness, absorption, absence of residue, etc.), benefits for the skin (moisturizing, softness, flexibility, relief of itching, etc.), form of presentation, product acceptance, purchase intention and scoring versus the products commonly used by each individual. The questionnaire was completed at the end of the study. The responses were grouped into two categories: favorable for the product (grouping of “agree” and “quite agree”) and unfavorable for the product (grouping of “quite disagree” and “disagree”). The users of products of this kind were asked about aspects related to satisfaction with the experimental product versus their usual product, and their purchasing intention.
In-use test in the pediatric population
The study was conducted in healthy pediatric volunteers (children and infants) of both sexes, after obtaining parental permission. The inclusion criteria were: age from 3 months to 18 years (3 or 4 babies under 3 years of age), Caucasian origin, indifferent body skin type, experiencing itching regularly, having sensitive body skin having sensitive skin and atopic-prone body skin. Exclusion criteria were: current use of other emollients or cosmetics in the application area in the last 14 days, subjects with acute dermatological processes, subjects taking anti-inflammatory and/or anti-allergic medication in the last 14 days, allergy or intolerance to cosmetic products or any of the components of the investigational product, subjects with lesions or skin spots in the application area. The participants were required to apply the minimum amount necessary to cover the entire body with the investigational product at home twice daily (morning and evening), for 28 days. The participants or their parents were provided with the normal instructions for use established by the manufacturer (apply to clean, dry skin and massage until completely absorbed). Skin acceptability was evaluated based on the clinical dermatological assessment, and cosmetic acceptability and subjective satisfaction were evaluated using a questionnaire.
Skin acceptability
Skin acceptability was assessed from clinical examinations by dermatologists on days 1 and 29. The specialists evaluated the appearance of skin signs after application of the product during 28 days. Acceptability was analyzed by calculating the frequency of skin signs and reactions to the product. Consideration was also made of the frequency of subjective responses regarding the absence of skin irritation (agree / quite agree / quite disagree / disagree) recorded from the question “The product does not irritate your skin”, included in a questionnaire completed by the participants at the end of the study.
Cosmetic acceptability and satisfaction
Cosmetic acceptability and satisfaction of the participants with the product were assessed based on the subjective responses of the subjects to specific questions about cosmetic aspects (pleasantness, texture, smell, ease of application, stickiness, absorption, absence of residue, etc.), benefits for the skin (moisturizing, softness, flexibility, relief of itching, etc.), form of presentation, product acceptance, purchase intention and scoring versus the products commonly used by each individual. The questionnaire was completed by the parents at the end of the study. The responses were grouped into two categories: favorable for the product (grouping of “agree” and “quite agree”) and unfavorable for the product (grouping of “quite disagree” and “disagree”). The users of products of this kind were asked about aspects related to satisfaction with the experimental product versus their usual product, and their purchasing intention.
Adult population
A total of 24 subjects were initially recruited, of which 22 started the study. Follow-up of three participants could not be made, so the final number of subjects for the analysis of results was 19 healthy volunteers between 22-70 years of age (mean age 56.2 years; 95% women). All the participants had a history of sensitive skin, and three of them (16%) reported a history of cosmetic reactions.
Skin acceptability
The investigational product showed very good skin acceptability in all the participants in the study. No clinical evidence of skin irritation was recorded by the dermatologist after four weeks of application. None of the subjects reported having experienced or observed any skin reaction in the form of irritation or discomfort during the study.
Cosmetic acceptability and satisfaction
With regard to the cosmetic efficacy and acceptability of the investigational product, a positive impression was recorded, particularly in reference to texture, applicability and efficacy of the product (Table 1).
|
Question |
Affirmative answer |
|
The appearance of the product is pleasant |
95% |
|
The product is pleasant for your skin type |
100% |
|
The texture of the product is pleasant |
89% |
|
The product has a light texture |
84% |
|
The smell of the product is pleasant |
100% |
|
The application of the product is easy and even |
95% |
|
The product nourishes the skin |
100% |
|
The product does not leave a sticky feeling on the skin |
84% |
|
The product spreads easly |
95% |
|
The product is quickly absorbed |
89% |
|
The product provides softness and suppleness from the first application |
100% |
|
The product provides softness, suppleness and hydratation for 12 hours after application |
100% |
|
The product is skin-friendly |
100% |
|
The product soothes the skin |
100% |
|
The product leaves a moisturising sensation |
100% |
|
The product leaves no white residue on your clothes |
84% |
|
The product leaves no greasy feeling |
89% |
|
The product improves the hydratation of your skin |
100% |
|
The product is suitable for daily use |
100% |
|
The product is suitable for use all over the body |
100% |
|
The product does not irritate the skin |
95% |
|
The product relieves itching |
100% |
|
The product reduces redness |
100% |
|
The packaging is suitable for correct application of the product |
100% |
|
The 500 mL format is suitable for daily use |
100% |
|
The product has met my expectations |
89% |
Table 1 Questionnaire on cosmetic acceptability and satisfaction in the adult population
The regular users of this type of product (17 participants) considered the results to be equal to or better than those obtained with their generally used product. Likewise, 65% preferred the investigational product over their usual product, and considered it to be more effective (Figure 1). Indeed, 89% of the participants intended to purchase it.
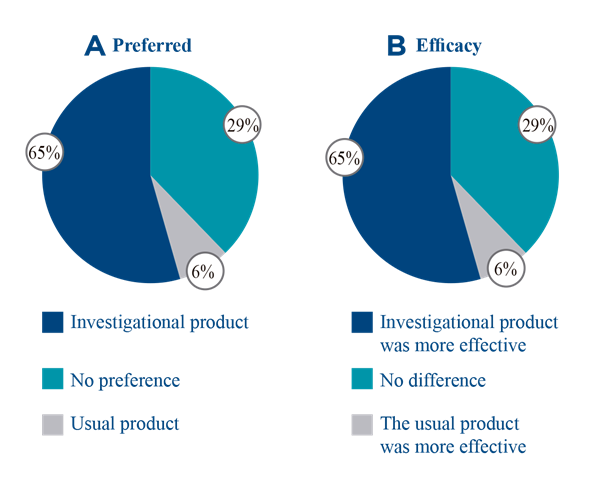
Figure 1 Questionnaire on satisfaction with regular users of emollients in the adult population. 1A. Percentage of answers to the question "What is your preferred product?" 1B. Percentage of answers to the question “Compared to your usual product, is the efficacy of the investigational product…?
Effect on the skin barrier from the TEWL values
After two weeks of repeated applications of the investigational product, a nonsignificant change in transepidermal water loss was observed versus the baseline values (10.96 ± 0.99 vs. 9.50 ± 0.68; p ≥0.10). In contrast, a statistically significant decrease in TEWL was observed after four weeks of repeated applications (10.96 ± 0.99 vs. 7.87 ± 0.41; p < 0.001) (Figure 2).
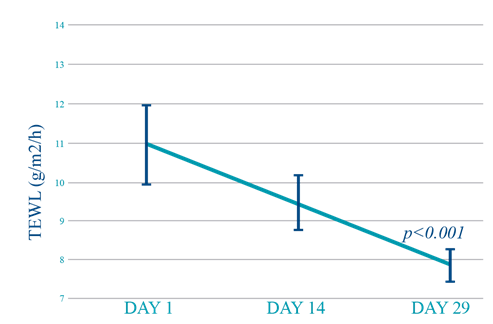
Figure 2 Transepidermal water loss in the adult population. Both values are compared against the initial value.
Pediatric population
A total of 30 subjects were initially recruited, of which 22 started the study. Follow-up of two participants could not be made, so the final number of subjects for the analysis of results was 20 healthy volunteers between 7 months and 16 years of age (mean age 7.3 years; 45% females). All the participants had a history of sensitive skin, and two of them (10%) reported a history of cosmetic reactions.
Skin acceptability
The investigational product showed very good acceptability in almost all the study participants (95%). The exception was a single subject with sensitive body skin and an atopic predisposition, who reported having experienced itching of mild intensity in the popliteal fossa of the knees, lasting a few seconds, immediately after each application of the product. Despite this, no clinical signs of skin irritation or intolerance were seen by the pediatrician at the end of the four weeks of application.
Cosmetic acceptability and satisfaction
With regard to the cosmetic efficacy and acceptability of the investigational product, a positive impression was recorded, particularly in reference to texture, applicability and efficacy of the product (Table 2). All of the regular users of this type of emollient product considered the results to be equal to or better than those obtained with their generally used product. Likewise, 42% preferred the investigational product over their usual product, and considered it to be more effective (Figure 3). Indeed, 95% of the participants intended to purchase it.
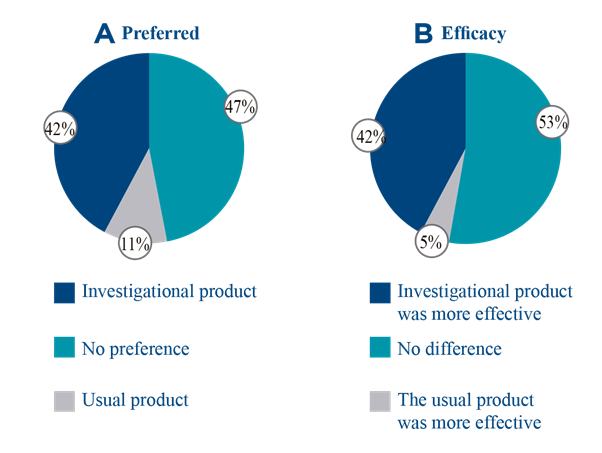
Figure 3 Questionnaire on satisfaction with regular users of emollients in the pediatric population. 1A. Percentage of answers to the question "What is your preferred product?" 1B. Percentage of answers to the question “Compared to your usual product, is the efficacy of the investigational product…?
|
Question |
Affirmative answer |
|
The appearance of the product is pleasant |
100% |
|
The product is pleasant for your skin type |
100% |
|
The texture of the product is pleasant |
100% |
|
The product has a light texture |
95% |
|
The smell of the product is pleasant |
100% |
|
The application of the product is easy and even |
100% |
|
The product nourishes the skin |
100% |
|
The product does not leave a sticky feeling on the skin |
90% |
|
The product spreads easly |
100% |
|
The absorption of the product is fast |
100% |
|
The product provides softness and suppleness from the first application |
90% |
|
The product provides softness, suppleness and hydratation for 12 hours after application |
95% |
|
The product is skin-friendly |
100% |
|
The product soothes the skin |
100% |
|
The product leaves a moisturising sensation |
100% |
|
The product does not leave residue on your clothes |
100% |
|
The product leaves no greasy feeling |
95% |
|
The product improves the hydratation of your skin |
100% |
|
The product is suitable for daily use |
100% |
|
The product is suitable for use all over the body |
100% |
|
The product does not irritate the skin |
100% |
|
The product relieves itching |
90% |
|
The product reduces redness |
100% |
|
The packaging is suitable for correct application of the product |
95% |
|
The format is suitable for daily use |
95% |
|
The product has met my expectations |
100% |
|
Globally, I liked the product |
100% |
Table 2 Questionnaire on cosmetic acceptability and satisfaction in the pediatric population
Atopic dermatitis is a chronic, relapsing inflammatory skin disorder characterized by severe itching, dryness and clinical manifestations such as edema and erythematous, scaly plaques that may be associated with lichenification.23 According to the published therapeutic guides, the treatment of AD should be based on the severity of the condition and the age of the patient, and includes both pharmacological treatment and non-pharmacological measures.41 The treatment options for flare-ups have been extensively studied.42 Although some medical treatments are available for preventing relapse, in addition to probiotics (the benefits of which are subject to debate), the use of emollients remains one of the best preventive strategies.43 Emollients are considered to be the essential basic component of any treatment, as they are effective in preventing flare-ups and in reducing the symptoms of AD, and moreover may contribute to reduce the use of corticosteroids.41,44–46 The clinical symptoms are correlated to increased transepidermal water loss and skin hypersensitivity, leading to alteration of the skin barrier.23
In assessing skin barrier function, the determination of TEWL is the most widely used objective measurement.47 The present study objectively analyzed skin parameters using the TewameterTM 300 device (Courage + Khazaka electronic), which is a widely accepted tool for the assessment of skin conditions. No statistically significant changes in TEWL were observed between the start of the study and the two weeks of application of the investigational product (p >0.10). However, greater improvement in this parameter was seen after four weeks of continued use of the product versus the start of the study (p <0.001). This means that the new body lotion, based on octenidine and silver citrate and especially designed for people with atopic-prone skin, proved useful in preventing transepidermal water loss after four weeks of repeated application, thereby improving skin barrier function. Although similar improvements in TEWL have been reported with the use of other therapies based on plant or herbal formulations, no studies have been made involving the topical application of a formulation similar to that of the investigational product.48 The control of AD and restoration of the skin barrier should be achieved as soon as possible, since this may help prevent the development of more severe and chronic forms of AD, particularly in infants. Further studies are needed, however, since there is some controversy in this field.49,50
With regard to skin acceptability, no abnormal clinical signs such as irritation or discomfort were observed by the dermatologist after four weeks of application. None of the subjects reported having had or observed skin reactions such as irritation or discomfort. The exception was a single subject in the pediatric group with sensitive body skin and an atopic predisposition, who reported having experienced itching of mild intensity in the popliteal fossa of the knees, lasting a few seconds, immediately after each application of the investigational product. This manifestation was regarded as probably related by the investigating pediatrician, though it did not lead to any change in the treatment regimen. The overall frequency of adverse reactions (as confirmed by the dermatologist) was 0%, allowing the product to be classified as “very well tolerated”, based on a comparison against approximately 2,406 products of the same kind (body lotion) controlled since 2004, and justifying the statement: “proven tolerance under pediatric control”.
Patient satisfaction with a treatment is a vital factor for its continued use.51 When asked about the cosmetic efficacy and acceptability of the investigational product, a positive impression was recorded among the participants, particularly in reference to texture, applicability and efficacy of the product (Tables 1 and 2). These results were particularly notorious among the pediatric patients, where 100% of the participants stated that the product met their expectations. This evidences high acceptance by the users of the new body lotion based on octenidine and silver citrate. Among the regular users of this type of product, 100% felt the skin effects of the investigational formulation to be "as good as" or "better" than those of the generally used product. Likewise, approximately 95% of the participants considered the investigational product to be as effective or even more effective than the commonly used product.
The data obtained confirm the good skin acceptability of the new cosmetic emollient after repeated applications under normal conditions of use, for 4 consecutive weeks. Likewise, the results show that a daily care and hygiene routine with the product based on octenidine and silver citrate, especially designed for people with atopic-prone skin and aimed at maintaining and restoring the structure of the epidermis, results in objective improvement of skin barrier function and a subjective perception of improved skin condition. Consequently, this over-the-counter cosmetic formulation with emollient and antipruritic properties is easy to use and relatively fast acting, containing octenidine and silver citrate, and could afford benefit in terms of the relief and control of atopic dermatitis.
None.
Authors declare that there is no conflict of interest.

©2023 Gairí, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.