eISSN: 2379-6367


Research Article Volume 12 Issue 5
1Heal Africa Hospital 111 Lyn Lusi, Goma, North Kivu; D.R. Congo
2Statistician Hugues C Masheka, 2Guys Logistics, Kampala Uganda
Correspondence: Pascal Gisenya, Heal Africa, Hospital 111 Lyn Lusi, Goma, North Kivu, D.R. Congo, Statistician Hugues C Masheka, 2Guys Logistics, Kampala Uganda, Tel +1 587 9747611
Received: September 16, 2024 | Published: September 30, 2024
Citation: Gisenya P, Paluku J, Kahatwa S. High burden on tuberculosis treatment for society-a survey study. Pharm Pharmacol Int J. 2024;12(5):179-183. DOI: 10.15406/ppij.2024.12.00449
Keywords: artemisia afra, resistant tuberculosis, non-resistant tuberculosis, costs
With an expected 10 million new cases and 1.6 million fatalities recorded every year since 2018, tuberculosis continues to have a significant negative impact on global health.1
During the last 2 decades, tuberculosis, an infection caused by Mycobacterium Tuberculosis has been the leading cause of death worldwide.1
During the Stop TB Partnership’s Global Plan to End TB, 2018–2022 (the Global Plan) it was estimated that US$ 8.6 billion was needed for TB programs worldwide for a total budget of 22 billion in 2022.1 According to the WHO report in 2023 the following figures were highlighted: an estimated US$ 2 billion per year were needed for TB research, US$ 13 billion per year for TB prevention, diagnostic and treatment services by 2022.2
It is of high importance to notice that many people who develop tuberculosis disease experience high burden of substantial economic costs during the disease episode and heavy losses while they are bedridden.2,3
Moreover, the ongoing difficulties that TB treatment poses, such as medication resistance (MDR-TB), treatment noncompliance call for a global effort to investigate novel treatment choices.
The objective of this study is to highlight deaths, and the different costs associated with the disease and propose a possibility to drastically shorten the length of treatment thus proving our case for a cheaper and safer solution.
|
Number of deaths non-resistant |
Number of deaths resistant |
|
|
2018 |
1.40 million |
2.40 million |
|
2019 |
1.32 million |
2.32 million |
|
2020 |
1.37 million |
2.37 million |
|
2021 |
1.39 million |
2.39 million |
|
2022 |
1.40 million |
2.40 million |
|
2023 |
1.50 million |
2.48 million |
|
2024 |
1.55 million |
2.50 million |
|
2025 |
1.58 million |
2.54 million |
|
2026 |
1.60 million |
2.62 million |
|
2027 |
1.64 million |
2.65 million |
|
2028 |
1.70 million |
2.70 million |
|
2029 |
1.75 million |
2.75 million |
|
2030 |
1.80 million |
2.85 million |
Table 1 Number of deaths non-resistant & resistant
|
Cost non-resistant |
Cost resistant |
|
|
2018 |
US$ 5.5 billion |
US$ 2.7 billion |
|
2019 |
US$ 6.4 billion |
US$ 3.8 billion |
|
2020 |
US$ 7.0 billion |
US$ 5.6 billion |
|
2021 |
US$ 7.0 billion |
US$ 7.0 billion |
|
2022 |
US$ 7.5 billion |
US$ 7.5 billion |
|
2023 |
US$ 7.7 billion |
US$ 9.0 billion |
|
2024 |
US$ 7.8 billion |
US$ 11.0 billion |
|
2025 |
US$ 8.0 billion |
US$ 13.5 billion |
|
2026 |
US$ 8.5 billion |
US$ 15.7 billion |
|
2027 |
US$ 8.8 billion |
US$ 17.5 billion |
|
2028 |
US$ 9.0 billion |
US$ 19.0 billion |
|
2029 |
US$ 9.5 billion |
US$ 20.5 billion |
|
2030 |
US$ 10.0 billion |
US$ 22.0 billion |
Table 2 Cost non-resistant & resistant
The comparative tables and graphs below show the advantages of using our solution (WHO drugs +artemisia Afra infusion) versus WHO drugs alone in terms of number of deaths reduction and substantial gains in terms of costs. (Table 3, Figure 3) & (Table 4, Figure 4).
|
WHO Alone |
WHO + Artemisia |
|
|
2018 |
3.80 million |
N/A |
|
2019 |
3.65 million |
N/A |
|
2020 |
3.74 million |
N/A |
|
2021 |
3.78 million |
N/A |
|
2022 |
3.80 million |
N/A |
|
2023 |
3.98 million |
N/A |
|
2024 |
4.05 million |
N/A |
|
2025 |
4.12 million |
0 |
|
2026 |
4.22 million |
0 |
|
2027 |
4.29 million |
0 |
|
2028 |
4.40 million |
0 |
|
2029 |
4.50 million |
0 |
|
2030 |
4.65 million |
0 |
Table 3 Number of deaths WHO alone &WHO + Artemisia
|
WHO alone |
WHO + Artemisia |
|
|
2018 |
US$ 8.2 billion |
N/A |
|
2019 |
US$ 10.2 billion |
N/A |
|
2020 |
US$ 12.6 billion |
N/A |
|
2021 |
US$ 14.0 billion |
N/A |
|
2022 |
US$ 15.0 billion |
N/A |
|
2023 |
US$ 16.7 billion |
N/A |
|
2024 |
US$ 18.8 billion |
N/A |
|
2025 |
US$ 21.5 billion |
US$ 1.8 billion |
|
2026 |
US$ 24.2 billion |
US$ 1.6 billion |
|
2027 |
US$ 26.3 billion |
US$ 1.5 billion |
|
2028 |
US$ 28.0 billion |
US$ 1.3 billion |
|
2029 |
US$ 30.0 billion |
US$ 1.2 billion |
|
2030 |
US$ 32.0 billion |
US$ 1.0 billion |
Table 4 Cost WHO alone &WHO + Artemisia
The costs associates with tuberculosis treatment are due to the inability to work thus reducing the productivity and other expenses linked to treatment which can take up to 12 months in case of resistance tuberculosis. In most case, especially in the developed countries, the governments take care of the disease management. However, several studies have shown that nevertheless the patients incur other expenses: transport, lodging, meals etc.4-9
Another burden to society is because most affected people by this disease are working age adults who are predominantly from poor households with limited financial resources. Most of the time, especially in Africa, this becomes a serious burden to families and in developed countries to governments (through welfare programs) and insurance companies as they must compensate the patients during the time spent at the hospital and when recovering from illness after treatment.
In this study we are going to analyze the costs associated with tuberculosis treatment (see tables and figures below for details).
It is our belief that by expanding the pilot study undertaken in 2023 and in 2024 from a small number (200) to a larger cohort (1000) patients we will be able to confirm our hypothesis for TB eradication worldwide,10–12 in 1 year (Figures 5–9) (Tables 5–8).
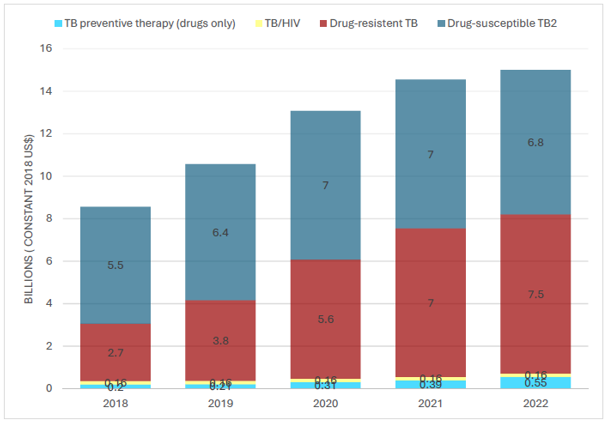
Figure 9 Estimates of funding required for TB prevention, diagnostic and treatment services in 128 low- and middle-income countries, a in the Global Plan to End TB 2018–2022.10
|
Direct medical |
Direct non-medical |
Indirect |
Overall |
|
|
WHO world region |
||||
|
African region |
293 (169–487) |
694 (460–995) |
807 (498–1266) |
1794 (1342–2431) |
|
Region of the Americas |
62 (26–135) |
174 (87–313) |
247 (111–506) |
484 (296–792) |
|
Eastern Mediterranean region |
103 (58–176) |
201 (136–283) |
212 (130–348) |
516 (395–684) |
|
European region |
32 (10–82) |
41 (27–58) |
56 (33–96) |
129 (77–217) |
|
South-East Asian region |
370 (201–644) |
825 (534–1210) |
899 (546–1424) |
2094 (1563–2795) |
|
Western Pacific region |
302 (113–743) |
410 (281–562) |
598 (321–1074) |
1310 (790–2137) |
|
All countries* |
1161 (642–2077) |
2345 (1601–3246) |
2820 (1846–4203) |
6327 (4583–8770) |
Table 5 Non-resistant tuberculosis
|
WHO ALONE |
WHO + ARTEMISIA |
|
|
African region |
1794 (1342–2431) |
299 (224–405) |
|
Region of the Americas |
484 (296–792) |
81 (49–132) |
|
Eastern Mediterranean region |
516 (395–684) |
86 (66–114) |
|
European region |
129 (77–217 |
21.5 (12–36) |
|
South-East Asian region |
2094 (1563–2795) |
349 (260–465) |
|
Western Pacific region |
1310 (790–2137) |
218 (131–356) |
|
All countries* |
6327 (4583–8770) |
1054 (763–1461) |
Table 6 Non-resistant tuberculosis
|
Direct medical |
Direct non-medical |
Indirect |
Overall |
|
|
African region |
22 (12–41) |
104 (63–164) |
108 (60–180) |
234 (178–306) |
|
Region of the Americas |
5 (2–10) |
25 (14–46) |
28 (13–54) |
58 (43–81) |
|
Eastern Mediterranean region |
8 (4–14) |
31 (19–50) |
25 (13–45) |
64 (46–87) |
|
European region |
40 (10–103) |
104 (69–154) |
110 (56–207) |
254 (181–382) |
|
South-East Asian region |
27 (14–47) |
119 (77–174) |
99 (62–154) |
245 (180–319) |
|
Western Pacific region |
24 (8–59) |
63 (44–90) |
71 (37–130) |
158 (113–237) |
|
All countries* |
126 (58–241) |
446 (304–647) |
441 (271–713) |
1013 (773–1355) |
Table 7 Resistant tuberculosis
|
|
WHO Alone |
WHO + artemisia |
|
|
African region |
|
234 (178–306) |
39 (29.66–51) |
|
Region of the Americas |
|
58 (43–81) |
9.66 (7.16–13.5) |
|
Eastern Mediterranean region |
|
64 (46–87) |
10.66 (7.66–14.5) |
|
European region |
|
254 (181–382) |
42.33 (30.16–63.66) |
|
South-East Asian region |
|
245 (180–319) |
40.83 (30–53.16) |
|
Western Pacific region |
|
158 (113–237) |
26.33 (18.83–39.5) |
|
All countries* |
|
1013 (773–1355) |
168.83 (128.83–225.83) |
Table 8 Resistant tuberculosis WHO alone &WHO + Artemisia
The costs linked to the tuberculosis treatment are divided between the direct costs: (hospital related i.e. diagnostic, laboratory, medications) and indirect costs: income lost, transportation of medications from central warehouses to hospital facilities and ultimately to patients.
Moreover, multidrug-resistant tuberculosis (MDR-TB) treatment is lengthy and may cause side effects which can lead to treatment drop out and establishment of drug-resistant strains.
Additionally, the intricacy of TB therapy necessitates a multi drug strategy creating logistical difficulties in low resources countries.
Finally, all the points mentioned above cause a significant cost by governments, insurance companies and at the end of the day the taxpayer must pay for it one way or the other.
The current Global Plan, for 2023–2030, estimates much higher funding needs, of US$ 15–32 billion per year in LMICs; this includes funding for implementation of a new TB vaccine after 2027. The political declaration adopted at the second UN high-level meeting on TB, held in September 2023, includes funding targets to mobilize US$ 22 billion per year by 2027 for TB diagnostic, treatment and prevention services, and US$ 35 billion per year by 2030; a target of US$ 5 billion per year by 2027 was set for investment in TB research.
We have demonstrated that the number of deaths has been increasing since 2018 and is not going down mainly because of resistant cases and the burden to society is alarming in terms of cost and unnecessary suffering. In 3 different clinical trials, the heal Africa research team on tuberculosis has demonstrated that artemisia Afra infusions given in combination with the standard WHO pills can shorten the duration of treatment on 105 people in less than 2 months10,11 and even on resistant cases the same treatment can do wonders by shortening the long treatment up to 12 or more to merely maximum 60 days.12
We are aiming to extend this pilot study on 1000 patients to plead our case on a larger cohort thus demonstrating that our study is unbiased by using rigorous scientific studies. We have also shown with sufficient data extracted from multiple studies published mainly by WHO and the Lancet that there is a huge burden to society in terms of costs, human suffering and unnecessary deaths that can be avoided by embracing this new opportunity that uses a natural plant extremely cheap and with no known side effects.
We strongly believe that the integration of Artemisia Afra into TB treatment strategy will represent a synergistic blend of traditional wisdom and modern science.13 It is therefore our hope that people of good will may accompany us financially and practically to demonstrate what we have observed on a few people to a larger cohort to address this huge burden to society and humanity.
None.
There is no conflict of interest in this survey and all the data are extracted from WHO publication and the Lancet. The authors declare that there is no conflict of interest.
None.

©2024 Gisenya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.