eISSN: 2379-6367


Research Article Volume 2 Issue 6
1Department of Pharmaceutical Technology, Asia Metropolitan University, Malaysia
2Department of Science, Technology and Engineering La Trobe University, Australia
Correspondence: Prabakaran Lakshmanan, Department of Pharmaceutical Technology, Faculty of Pharmacy, Asia Metropolitan University, Batu-9, Cheras, Selangor, Malaysia , Tel 60183927169
Received: June 16, 2015 | Published: November 23, 2015
Citation: Kaur S, Lakshmanan P. Hibiscus Rosa-sinesis leaves polysaccharide as solubility enhancing agent for poorly soluble drug. Pharm Pharmacol Int J. 2015;2(6):189-195. DOI: 10.15406/ppij.2015.02.00040
The main purpose of this research was to use hydrophilic Hibiscus Rosa-sinesis (HRS) dry leaves mucilage as functionality solubility enhancing agent for the design and development of solid dispersion of the drug Telmisartan (TS) to formulate tablets and to study its in vitro quality control tests. The mucilage was extracted, isolated, purified and prepared solid dispersions with different ratio of drug: mucilage (1:0.5, 1:1.0, 1:1.5, 1:2.0 and 1:2.5) using solvent evaporation method and another. Two formulations were prepared using drug: mucilage ratio of 1:1.0 as co-grinding method (GM) and physical mixture (PM). The solubility study showed a significant enhancement in the aqueous solubility of the drug where the solubility rate was increased with the increment in ratio of the mucilage. The formulation giving the best solubility enhancement of Telmisartan in aqueous media was in the order of SD>GM>PM>API. Then, the dispersions were compressed into tablets where it passed the pre-compression and post-compression evaluation parameters. Swelling index increased as the amount of mucilage added to each formulation (SD1 to SD3). Nevertheless as the amount of mucilage is increased for formulation SD4 and SD5 (1:2.0 and 1:2.5 ratio of drug: mucilage) the swelling index were decreased. The in-vitro dissolution study revealed that there is marked decrease in the dissolution rate of Telmisartan for all the solid dispersion containing tablets when compared to Telmisartan API tablets. This proves with the increase in mucilage ratio, the drug release from the solid dispersion tablet got retarded because of the formation of highly viscous barrier layer at the interface of the tablet and dissolution medium. In conclusion, the solubility of the drug has been greatly enhanced by the solid dispersion technique incorporating HRS mucilage using solvent evaporation method. Therefore, it is suggested that the drug Telmisartan can be converted to solid dispersion using Hibiscus Rosa-sinesis dry leaves mucilage as solubility enhancing agent and the solid dispersion granules can be filled in capsules as a dosage form.
Keywords:hybiscus leavespolysaccharide, telmisartan, solubility, solid dispersion, solvent evaporation, co-grinding technique
TS: Telmisartan; API: Active Pharmaceutical Ingredient; SD: Solid Dispersion; GM: Ground Mixture; PM: Physical Mixture; AR: Analytical Reagent; DM Water: Demineralised Water; MCC: Microcrystalline Cellulose; HRS: Hybiscus Rosa Sinensis
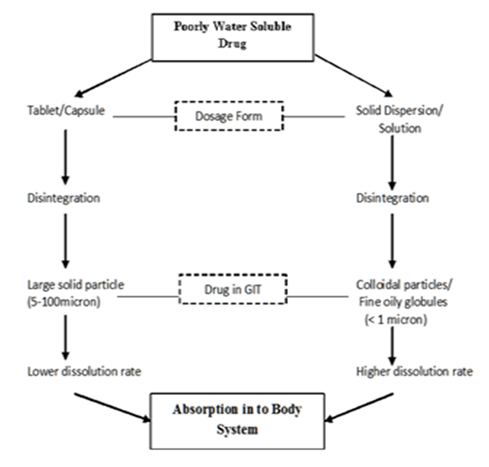
Oral drug delivery is the most convenient and preferred route of administering drugs owing to its greater stability, smaller bulk, correct dosage and easy production.1 Nevertheless, the real problem faced with the design of oral dosage forms are related to the drug poor bioavailability that is mainly caused by poor solubility and low permeability.2 Hence, one of the main challenges for pharmaceutical industry is associated to approaches of improving the water solubility of drugs generally for oral-drug delivery system.1 There are certain aspects to be considered when selecting the suitable solubility enhancement techniques like the properties of drug under consideration, nature of excipients to be selected and nature of intended dosage form.2 Solid dispersion technique is one of the most extensively studied and successful strategy to improve the drug release of poorly soluble drugs and it is a molecular mixtures of poorly water soluble drugs in hydrophilic carriers or matrix, which present a drug release profile that is driven by the polymer properties.1,3 The dissolution rate and bioavailability of poorly soluble drugs are increased as this technique works by the means of reducing particle size to nearly almost of molecular level which is illustrated in Figure 1.4
In recent times, plant derived polymers like gums and mucilages are gaining more interest as they fulfill many requirements of pharmaceutical excipients because they are non-toxic, stable, easily available, capable of chemical modifications, potentially biodegradable and inexpensive.5Hibiscus Rosa-sinesis Linn which is the red flowered variety is preferred in medicine as the leaves and flowers are shown to be promoters of hair growth and helps in wound-healing.6 The leaves of the hibiscus species have plenty of water soluble mucilage that can produce aqueous colloidal suspension when make contact with water.7 This mucilage will be suitable as an excipient in an formulation when poorly soluble drug are incorporated, it brings them to contact with water faster which would facilitates the improvement of drug solubility.
Telmisartan is 2-(4-{[4-methyl-6-(1-methyl-1H-1,3-benzodiazol-2-yl)-2-propyl-1H-1,3-benzodiazol-1-yl]methyl}phenyl) benzoic acid.8 It is angiotensin II receptor blocker (ARB), which is used in the prevention and treatment of hypertension.9 The main problem encountered with Telmisartan is the poor solubility in biological fluids that effects into poor bioavailability after oral administration of 42% giving late onset of action. Therefore, the present work aimed, to improve the water solubility of Telmisartan especially for its oral-drug delivery system, solid dispersion technique was applied with the choice of a natural polymer that can enhance the bioavailability. Hibiscus rosa-sinesis leaves mucilage is chosen as the natural polymer because have hydrophilic character and high swelling capacity. In addition, this leaf mucilage are freely available and the water soluble substance of this leaf extract that generally goes to waste would be beneficial.
Telmisartan API was procured from Dr Reddy’s Laboratories, India as gift sample. The Hybiscus leaves (red flower) were collected from the garden of Housing Area, Batu 9, Cheras, Selangor, Malaysia and the water soluble mucilage were extracted from Asia Metropolitan University Pharmaceutical Technology Lab. The other chemicals and solvents were of AR grade. The other chemicals and solvents were of AR grade purchased from an authenticated vendor in Malaysia. Dissolution test apparatus - Electrolab, India, UV spectrophotometer - Shimadzu, Japan, Tap density apparatus – Electrolab, India and other equipments were of ISI grade used for the study.
Preformulation study
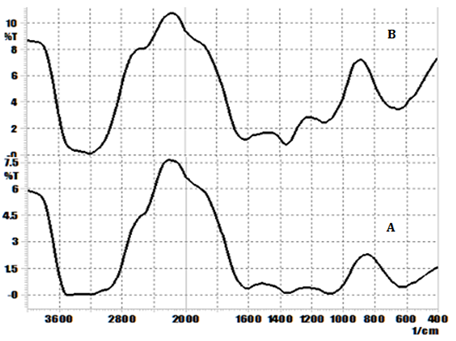
Fourier transform infrared spectroscopy (FTIR): The samples of mixture of drug, mucilage, microcrystalline cellulose, drug + mucilage and drug + mucilage + microcrystalline cellulose were checked for their compatibility which was evaluated by the use of Shimadzu FTIR 8400S Spectrophotometer (Shimadzu, Japan) by potassium bromide (KBr) pellet method with weight ratio of sample and KBr 2mg to 200mg using hydrostatic press at a force of 5t cm-2 for 5minutes. The spectral scanning was conducted from 4000 to 400 cm−1 at a resolution of 4 cm−1. The experiment was duplicated to check the reproducibility.10
Particle size distribution of dry water soluble mucilage: The mucilage powder was dispersed in light liquid paraffin in a clean glass slide and cover slipped. The microscopic study for the particle size was done using Motic photomicroscope (Motic Images plus Ver. 2.0). The images were captured at 10x objective lens. Around 500 particles were studied and measured for its size in micrometer.
Extraction of dry water soluble mucilage 7: The matured leaves from hibiscus species were collected, washed, dried at 37 °C for 24hr. Then it was crushed and soaked in warm demineralised (DM) water for 2-3hr and then left aside for 24 hr. Next day, the soaked leaves was stirred using overhead stirrer for 1-2 hr and it was heated up to 80-90 °C for 30-45 min while stirring for complete release of the water soluble mucilage/polysaccharide in to the solvent. The mucilage was extracted by using a muslin cloth bag to remove the marc and acetone was added to the concentrated viscous solution with constant stirring. The precipitate formed was then separated by filtration and washed 2-3 times with acetone until obtain the pale yellow course powder, dried in an oven at 37 °C, collected, grounded, passed through sieve (# 40) and stored in desiccator. The dry powder was considered as water soluble polysaccharide for pharmaceutical use.
Method of solid dispersion and tablets formulation
Preparation of telmisartan solid dispersion: Solid dispersions of Telmisartan were prepared using different ratio by weight of HRSdry leaves mucilage as shown in Table 1. Co-grinding method: Ground mixture is prepared by mixing Telmisartan and HRSdryleaves mucilage in mortar and pestle by triturating it for 20 minutes. The triturated mixture was then passed through sieve (# 20) and it was stored in a zipper bag at room temperature.11 Solvent Evaporation method: The weighed drug and mucilage were placed in a china dish and gradually added around 10ml of chloroform with constant mixing. The slurry mixture was then heated at 40 ºC for the complete removal of solvent. The solid dispersion obtained was then scraped out with a spatula and was pulverized in a mortar to get the dry free flowing powder. The powder was then passed through a sieve (# 20) and it was stored in a zipper bag at room temperature.9,12
Formulation of telmisartan sd tablets 13: Formulations of solid dispersion tablets as shown in Table 2 all weighing 220mg contains 40mg of Telmisartan that is dispersed with Hibiscus Rosa-sinesis leaves mucilage, also mixed properly with microcrystalline cellulose (MCC) as diluent, starch as disintegrating agent which was pre sieved (#60) and blended for 5 min to get complete distribution of active throughout the power mixture. The other excipients like talc and magnesium stearate is added finally for lubricating the powder mixture. The solid dispersion formulations (SD1 – SD5) are prepared using the direct compression method. The ground mixture formulation (GM) was prepared using the wet granulation method. All the powder blends were punched into tablets using 10 stations Karnavati GMP rotary tablet machine.
Pre and post formulation evaluation methods
Percentage practical yield of prepared solid dispersions of Telmisartan 12 : The prepared solid dispersions were collected and weighed accurately and the percentage practical yield was calculated by using the formula as follows:
Pre-compression parameters of granules 14
Angle of repose: The flow property of the powder blend was determined by fixed funnel method. (Where h = height of the powder cone, r = radius of the powder cone)
Tan θ = h/r
Bulk Density: Bulk density of each batch was determined by placing pre-sieved drug excipient blend in to a measuring cylinder and measuring the initial weight (M) and initial volume (Vb).
Db = M/ Vb
Tapped Density: The mechanical tapping of the powder for each batch in the measuring cylinder for bulk density was performed using tapped density tester (Electrolab tap density tester, USP) at a constant rate for 100 times. Tapping was continued until no further change in volume was noted. The volume is known as tapped volume (Vt).
Dt = M / Vt
Compressibility index (CI) and Hausner’s Ratio: Indicates the powder flow properties and measured for relative importance of inter-particulate interactions.
CI = Dt – Db / Dt × 100
Hausner’s ratio = Dt / Db
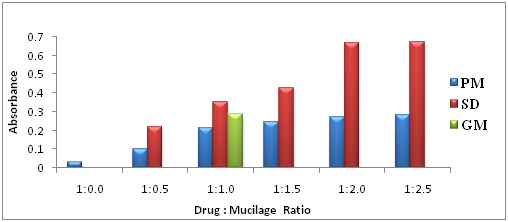
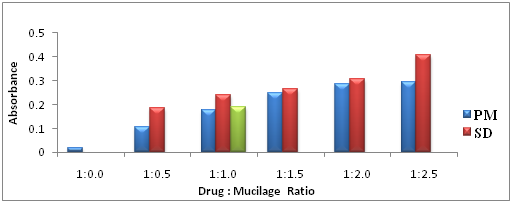
Solubility Study of Telmisartan physical mixtures and dispersions: The solubility study was conducted for the API Telmisartan, physical mixture (PM), ground mixture (GM) and solid dispersions (SD) of Telmisartan and the solvents/aqueous media used were distilled water, phosphate buffer pH 6.8 and phosphate buffer pH 7.4. Firstly, little amount of solvents are added in the 100ml volumetric flask containing the preparations of Telmisartan (100mg of drug and its equivalent) and allowed it for an hour for complete wet and swell, after that some more solvents was added and shaken well. Afterward, all the flasks were added approximately 50% of solvents and the flasks were intermittently shaken for 6hr. The flasks were left for 24hr at room temperature. Next day, the flasks were shaken for 5min and made the volume, filtered through Whatman filter paper of pore size 0.45µm. From this, 1ml of solution was withdrawn and diluted with 9ml of distilled water and samples were then scanned using UV spectrophotometer at 296nm, absorbance values are obtained and the solubility of the Telmisartan in all preparation were calculated and compared.
Post-compression parameters for Telmisartan solid dispersed tablets 15
Drug Content: Tablets from each batch was crushed and equivalent to 40mg of drug was dissolved in 100ml of 0.1N HCl in volumetric flask and it was allowed to dissolve the drug in the solvent. The solution was filtered and diluted with 0.1N HCl. The samples were then analyzed using UV spectrophotometer at 296nm and absorbance values are obtained. The concentration of Telmisartan was calculated by using standard calibration curve of the drug.
Weight variation test: Tablets were randomly selected from each batch after compression and weighed individually. The average weights of tablets were determined and calculated for its weight variation.
Hardness test: Tablets were randomly selected from each formulation and underwent hardness test by using Schleuniger hardness tester. The average hardness was determined.
Friability test: The friability of tablets was determined using Roche Friabilator. Tablets from each batch were initially weighed (W0) and transferred into friabilator. The friabilator was then operated at 25 rpm for 4 minutes or run up to 100 revolutions. The tablets were weighed again (W). The % friability was calculated.
Thickness test: The thickness of the tablets was determined by using Vernier caliper. Ten tablets were randomly selected from each batch and the average values were determined.
Swelling Index: The extent of swelling was measured in terms of % weight gain by the tablets. The swelling behavior of tablets from the entire batch was studied by randomly selecting tablets from each batch and the initial weight was noted. The swelling of tablet was determined by placing the tablets separately in a petri dish containing excess of 0.1 N HCl at the room temperature for 8 hr. At the end of 8th hr, the tablet was withdrawn and excess water was removed using tissue paper. The swollen tablets were then reweighed (final weight) and swelling index of each tablet was calculated using the formula as follows:
In-vitro Dissolution test 16: In-vitro release profile of solid dispersion tablets as well as pure drug tablets was performed using USP type-II apparatus (paddle) in 900ml of 0.1M HCl at 37±0.5 ˚C and the paddle rotation speed was set at 50rpm. At specified time period, 5ml of the sample was withdrawn and filtered through 0.45μm and replaced with the same volume of fresh dissolution medium in order to keep the total volume constant. The samples were analyzed for drug content using double beam UV-Visible spectrophotometer at 296nm. All samples were analyzed in triplicate.
Based on the FTIR spectrum (Figure 2) (Figure 3), the results revealed that there is no change in the peak identified in the particular wavelength indicating that there was no chemical interaction between drug, mucilage and excipients used in the study. Approximately 500 of particles was studied in particle size distribution study and the average, minimum and maximum size of the mucilage particles was found to be 7.52µm, 1.6µm and 47.18µm respectively. The dried powder which was obtained is white creamy powder, acceptable and characteristic odour, having mucilaginous taste and coarse powder. The mucilage powder was swelling well and soluble in water whereas it was not soluble in acetone and alcohol. The solvent was used to extract the mucilage is DM water and the percentage yield of the dry water soluble mucilage was 7.8%.
Telmisartan solid dispersions was done by solvent evaporation technique using isopropyl alcohol as dispersing liquid with the practical yield in the range between 90.00 to 99.76%. Solid dispersion tablets were also produced without any practical issues while using direct compression technique. But, the co-grinding mixture was in need of wet granulation (isopropyl alcohol was used as granulating fluid) of the mixture for direct compression. The pre and post compression parameters for the SD granules of all the batches were analysed like bulk density, tapped density, compresibility index (CI), Hausner’s ratio, angle of repose and drug content, weight variation, hardness, friability, thickness, swelling index results are shown respectively in Table 3 & Table 4.
The solubility of Telmisartan in aqueous media like distilled water, phosphate buffer pH 6.8 and phosphate buffer pH 7.4 increases with the increment in ratio of the HRS polysaccharide was illustrated in Figure 4 & Figure 5 respectively. Based on all the solubility graphs in the three aqueous media, it is proven that the preparation that showed the greatest solubility enhancement of Telmisartan is solid dispersion, followed by ground mixture and finally physical mixture when compared to the solubility of API Telmisartan alone. The better solubility rate of the solid dispersion formulations would have been due to improved wettability of the drug particles and considerable reduction in the particle size during the formation of solid dispersion. In addition, the solubility rate could have been enhanced due to increase in the surface area of drug Telmisartan, proper dispersion and increase in the amorphicity of drug by adsorption on the surface of mucilage. The elevated rate of solubility of the water solubleHibiscus Rosa-sinesis leaves mucilage as the component of solid dispersion would not only draw along more insoluble but finely mixed drug Telmisartan into aqueous media.
The in vitrorelease studies reveal that there is marked decrease in the dissolution rate of Telmisartan for all the solid dispersions when compared to pure Telmisartan itself. The dissolution rate of Telmisartan in the solid dispersion formulations was strongly dependent on the relative ratio of the polysaccharide. This proves with the increase in mucilage ratio, the drug release from the solid dispersed tablet got retarded. This is because by increasing the mucilage percentage or ratio in the solid dispersion formulation, a highly viscous barrier layer was formed between the interface of drug and dissolution medium. This highly viscous barrier layer may acts as the primary control for resisting to erosion and the diffusion of the drug Telmisartan.


S No. |
Formulation Code |
Drug : Mucilage Ratio |
Method |
1 |
Mucilage |
0.0 : 0.5 |
Blank |
2 |
API |
01:00.0 |
|
3 |
PM1 |
01:00.5 |
Physical Mixture |
4 |
PM2 |
01:01.0 |
|
5 |
PM3 |
01:01.5 |
|
6 |
PM4 |
01:02.0 |
|
7 |
PM5 |
01:02.5 |
|
8 |
SD1 |
01:00.5 |
Solvent Evaporation |
9 |
SD2 |
01:01.0 |
|
10 |
SD3 |
01:01.5 |
|
11 |
SD4 |
01:02.0 |
|
12 |
SD5 |
01:02.5 |
|
13 |
GM |
01:01.0 |
Co-Grinding |
Table 1 Drug: Mucilage ratio for various preparations of Telmisartan solid dispersion
Formulation Code |
SD1 |
SD2 |
SD3 |
SD4 |
SD5 |
GM |
Ingredients |
(1:0.5) |
(1:1.0) |
(1:1.5) |
(1:2.0) |
(1:2.5) |
(1:1.0) |
Telmisartan |
40 |
40 |
40 |
40 |
40 |
40 |
Hibiscus Rosa-Sinensis Leaves Mucilage |
20 |
40 |
60 |
80 |
100 |
40 |
Microcrystalline Cellulose |
105 |
85 |
65 |
45 |
25 |
85 |
Starch |
50 |
50 |
50 |
50 |
50 |
50 |
Magnesium Stearate |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
Talc |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
Total Weight of Tablet |
220 |
220 |
220 |
220 |
220 |
220 |
Table 2 Formulation of Telmisartan solid dispersion tablets
No. |
Formulations |
Bulk Density (g/ml) |
Tapped Density (g/ml) |
CI (%) |
Hausner’s Ratio |
Angle of Repose (θº) |
1 |
SD1 |
0.480 |
0.600 |
20.00 |
1.250 |
29.7 |
2 |
SD2 |
0.461 |
0.585 |
21.20 |
1.269 |
28.9 |
3 |
SD3 |
0.471 |
0.600 |
21.50 |
1.274 |
29.1 |
4 |
SD4 |
0.461 |
0.585 |
21.20 |
1.269 |
28.9 |
5 |
SD5 |
0.471 |
0.600 |
21.50 |
1.274 |
28.2 |
6 |
GM |
0.436 |
0.558 |
21.86 |
1.280 |
33.2 |
Table 3 Pre-compression parameters of the SD granules
No. |
Formulations |
Drug Content (%) |
Average Weight (mg) |
Average Hardness (kg/cm2) |
Friability (%) |
Average Thickness (cm) |
Swelling Index (%) |
1 |
SD1 |
98.7 |
224 |
12.36 |
0.085 |
0.2 |
157.5 |
2 |
SD2 |
97.83 |
226 |
12.42 |
0.124 |
0.2 |
179.4 |
3 |
SD3 |
96.08 |
226 |
12.41 |
0.168 |
0.2 |
198.1 |
4 |
SD4 |
96.95 |
222 |
12.03 |
0.222 |
0.2 |
164.3 |
5 |
SD5 |
95.65 |
220 |
12.45 |
0.232 |
0.2 |
162.1 |
6 |
GM |
95.23 |
220 |
11.97 |
0.138 |
0.2 |
175.5 |
Table 4 Post-compression parameters of SD Telmisartan tablets
Solid dispersions of Telmisartan were succestully prepared and formulated by different techniques like solvent evaporation and co-grinding method by using the natural polymer Hibiscus rosa-sinesis dry leaves polysaccharide powder. A significant enhancement in the aqueous solubility of the drug Telmisartan was observed from the solubility study. The solubility rate of Telmisartan in aqueous media increases with the increment in ratio of the mucilage showing its potential role for pharmaceutical use as solubility enhancing agent. On the other hand, when the all the solid dispersions formulation was compressed into tablets, the in-vitro dissolution study done and the results revealed that there is marked decrease in the dissolution rate of Telmisartan for all the solid dispersion containing tablets when compared to pure Telmisartan tablets itself. This proves with the increase in mucilage ratio, the drug release from the solid dispersion tablet got retarded because of the formation of highly viscous barrier layer at the interface of drug Telmisartan and dissolution medium. Therefore, it is suggested that the drug Telmisartan can be converted to solid dispersion using Hibiscus Rosa-sinesis dry leaves mucilage as solubility enhancing agent and the solid dispersion granules can be filled in capsules and supplied as a dosage form and converting to tablet dosage form for sustained effect.
Authors are thankful to the Management, Asia Metropolitan University, Cheras, Malaysia for providing lab and other necessary facilities for carrying out this work, University Putra Malaya, Malaysia for providing lab facilities to perform sugar and fatty acid analysis, authors are also thankful to the lab in-charge and technicians for their support.
The author declares no conflict of interest.

©2015 Kaur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.