eISSN: 2379-6367


Research Article Volume 2 Issue 3
1Department of Anatomy and Cell Biology, Oklahoma State University-Center for Health Sciences, USA
2Department of Pharmaceutical Sciences, University of Oklahoma-Health Sciences Center, USA
3Department of Biotechnology, Tulsa Community College, USA
Correspondence: Heith A Crosby, Oklahoma State University-Center for Health Sciences, Department of Anatomy and Cell Biology, 1111 West 17th Street Tulsa, Oklahoma 74107, USA, Tel 918.561.5817
Received: February 26, 2015 | Published: June 16, 2015
Citation: Crosby HA, Ihnat M, Spencer D, et al. Expression of glutaminase and vesicular glutamate transporter type 2 immunoreactivity in rat sacral dorsal root ganglia following a surgical tail incision. Pharm Pharmacol Int J. 2015;2(3):86-93. DOI: 10.15406/ppij.2015.02.00023
Glutamate is an excitatory neurotransmitter, released by primary sensory peripheral nerve and spinal synaptic terminals during nociceptive (pain) signaling. The primary source of neurotransmitter, glutamate, is provided from its synthetic enzyme, glutaminase (GLS). Neurotransmitter glutamate is packaged into synaptic vesicles in nociceptive neurons by the vesicular glutamate transporter 2 (VGluT2). Little is known, however, what effect a surgical incision has on GLS and VGluT2 in primary afferent neurons. In this study, an aseptic, midline incision in the proximal one-third of the rat-tail was examined to determine whether sacral dorsal root ganglia innervate the area of surgical incision, utilizing the retrograde tracer Fluoro-Gold™. Subsequently, the amount of VGluT2 and GLS immunoreactivity (IR) in sacral dorsal root ganglia (DRG) was evaluated using immunofluorescence with image analysis and Western immunoblotting with density analysis. GLS messenger RNA (mRNA) changes were evaluated using real-time reverse transcriptase polymerase chain reaction (RT2-PCR). Our findings revealed that sacral-1 (S1) DRG neurons innervate the area of surgical incision. Both GLS and VGluT2-ir are elevated post-surgical incision in S1 DRG neurons for up to 72hours, while GLS mRNA levels rapidly decreased post-incision and remain depressed for at least 96hours. Following a surgical incision of the tail, sacral DRGs rapidly deplete their available supply of GLS mRNA and alter their production of the synthetic enzyme, GLS and the vesicular transporter, VGluT2. The rapid use of GLS mRNA and subsequent elevation of GLS protein, along with VGluT2 protein may result in both increased glutamate production and release at peripheral and central processes contributing to primary and secondary sensitization, respectively.
Keywords: glutaminase, glutamate, incision, post-surgical pain, VGluT2, DRG, pain, mRNA, PPP, GLS
AIA, adjuvant-induced arthritis; BCA, bicinchonic acid; BDNF, brain derived neurotrophic factor; CCD, charge-coupled device; DEP, diethylpyrocarbonate; DRG, dorsal root ganglion; ECL, enhanced chemiluminescence; GLS, glutaminase; IR, immunoreactivity; MGI, mean grayscale intensity; NGF, nerve growth factor; PBS, phosphate buffered saline; PBS-T, phosphate buffered saline with triton X-100; pH-RE, pH-response element; PPP, persistent post-surgical pain; PVP, polyvinylpyrollidone; ROI, regions of interest; RNA, ribonucleic acid; RT2-PCR, real-time reverse transcriptase polymerase chain reaction; S1, sacral 1; TBS, tris-buffered saline; VGluT2, vesicular glutamate transporter 2
Surgical procedures are common in the United States with estimates at greater than 45million per year and it is estimated that the average American undergoes 9.2surgical procedures in his/her lifetime.1 A serious clinical problem that can occur as a result of surgery is the condition of persistent postsurgical pain (PPP). This has been described and defined as pain that lasts longer than 3months after surgery. This chronic painful condition can affect as much as 50% of surgical patients, with up to 10% of the patients rating the pain as moderate to severe. Several surgical procedures (e.g., inguinal herniotomy, leg amputation, breast surgery and thoracotomy) have well documented persistent pain syndromes.2 The need to understand the underlying molecular neurobiology after a surgical procedure is necessary in hopes of identifying future pharmacological and treatment modalities.
Glutamate is the amino acid neurotransmitter used by primary sensory neurons of the dorsal root ganglion (DRG). Glutamate is produced by the mitochondrial metabolic enzyme, glutaminase (GLS) via the hydrolytic deamination of glutamine. Once produced, glutamate is packaged into synaptic vesicles by vesicular glutamate transporter 2 (VGluT2) and released from peripheral nerve terminals and spinal synaptic terminals during nociceptive signaling.3 GLS is elevated in both the cytoplasm and mitochondria of primary afferent sensory neurons in the adjuvant-induced arthritis (AIA) model in the rat. GLS first appears elevated in the DRG cytoplasm between 1-2days AIA followed by an increase in the mitochondria between 2-4days. Enzyme activity, protein and immunoreactivity (IR) remained elevated in the DRG neuronal cell bodies through days 2-8. GLS in peripheral nerve terminals in skin also are elevated during AIA. This elevation of GLS enzyme allows sensory nerves in the skin to produce increased amounts of glutamate during inflammation. Elevated glutamate production and release from sensory nerves contributes, in part, to the enhanced pain sensitivities of inflammation and helps maintain primary hyperalgesia.4,5 Based on this work, our lab examined the effect a surgical incision has on the levels of GLS and VGluT2 protein and GLS mRNA in the DRG. In this study, the following hypothesis is addressed: If GLS is elevated in DRG neurons during painful inflammatory conditions,5 then GLS levels will be elevated in the affected dorsal root ganglia following a surgical incision. The overall experimental goal was to determine the temporal expression of GLS in rat dorsal root ganglion post-surgical incision. Our lab first evaluated whether sacral 1 (S1) DRG neurons innervate the area of tail incision with retrograding tracing, followed by subsequent evaluation of the effect a surgical tail incision has on GLS- and VGluT2-ir and GLS mRNA in rat S1 DRG.
Animals
Harlan Sprague-Dawley rats (Oklahoma State University Center for Health Sciences breeding colony originating from Charles River) were housed on a 12-hour light: 12-hour dark cycle and given free access to food and water. Procedures were conducted according to guidelines from the National Institutes of Health6 and were approved by the Oklahoma State University Center for Health Sciences Institutional Animal Care and Use Committee. All appropriate efforts were made to minimize the number of animals used in these studies.
Surgical incision
Harlan Sprague-Dawley rats (n=125, male and female), weighing between 250 - 350 grams (mean weight=298.3grams, mean age=271.5days), were anesthetized with 5% isoflurane in oxygen (3 L/min) in a plastic induction chamber until mobility ceased. A nose cone was utilized to deliver maintenance anesthesia (isoflurane 2-3%, O2 1.5L/min). The tails of the rats were cleaned using providone iodine for disinfection of the surgical site. The experimental animals had a 20mm longitudinal incision made through skin, fascia and muscle on the dorsal surface of the tail. The incisions were made longitudinally, along the midline of the proximal third of the tail, with half the incision above and half below the proximal third midpoint. The incisions were sutured using a 19 mm reverse cutting needle with two 5.0 nylon sutures. Control animals were surgical naïve rats that underwent anesthesia and disinfection of the surgical site as described previously.
Retrograde tracer
Harlan Sprague-Dawley rats (n=6; male and female; 3 control, 3 experimental) were anesthetized and a 20mm surgical incision was made in the proximal one third of the tail as described previously. Controls were surgical naïve rats. Injections of the retrograde tracer, Fluoro-Gold™ (Flurochrome, LLC; Denver, Colorado), were made into either the surgical incision or the mid-point in the proximal one third of the tail in experimental and control animals, respectively.7 At 3days post-injection, rats were deeply anesthetized with tribromoethanol (2.5% w/v) (Sigma-Aldrich; Saint Louis, Missouri) plus xylazine (100mg/ml) (Lloyd Laboratories; Walnut, California) and perfused through the ascending aorta with 75mL calcium-free Tyrode’s solution (pH 7.3), followed by 325mL paraformaldehyde/picric acid fixative.8 Lumbar and sacral DRG were collected, post-fixed for 4hours (hr) and incubated in 10% sucrose in phosphate buffered saline (PBS), pH 7.4, overnight at 4°C. DRG tissue sections were cut at 20μm with a cryostat (Leica Microsystems; Buffalo Grove, Illinois). Sections were processed for Fluoro-Gold™ and VGluT2 (Sigma-Aldrich; Saint Louis, Missouri) immunofluorescence. Images of Fluoro-Gold™ and VGluT2 were collected with a BX51 microscope (Olympus; Waltham, Massachusetts) and SPOT RT740 camera (Diagnostic Instruments; Sterling Heights, Michigan) and evaluated with image analysis software (Image J, NIH; Bethesda, Maryland). The values for area (µm2) were binned into small (<400µm2), medium (400-799.99µm2) and large (>800µm2) size categories to indicate three broadly defined neuronal subpopulations that correspond to C-, Aδ and Aα/β fibers, respectively.8–10
RNA isolation
The following time points were analyzed: 1, 2, 4, 12, 24, 48 and 96 hours. Harlan Sprague-Dawley rats (n=95; male and female) underwent CO2 asphyxiation and S1 DRG’s were obtained by rapid dissection and placed in RNAlater™ (Qiagen; Germantown, Maryland).11 RNA was isolated from combined right and left S1 DRG cell lysates using the Origene Vantage Total RNA Kit™ (OriGene; Rockville, Maryland). Using fine-tip forceps, DRG tissue samples were transferred to micro-centrifuge tubes containing a cellular lysis buffer and homogenized promptly using a sterile Teflon® pestle. The homogenized lysate was centrifuged to pellet any debris. The supernatant was decanted into RNase-free micro-centrifuge tubes. Ethanol (70%) with diethylpyrocarbonate (DEPC) was added to the lysate. The lysate was vortexed and centrifuged (14,000g) for two minutes and the supernatant decanted onto an assembled RNA binding (spin) column and centrifuged (14,000g). The flow-through was discarded and wash buffer was added onto the spin column and centrifuged (14,000g) for two minutes. The previous wash step was repeated three times. Between each centrifugation, the spin column was placed into a dry collection tube. To elute the RNA, the spin column was placed into a 1.7mL micro-centrifuge recovery tube and RNA elution buffer was added. The sample was centrifuged for 10 minutes at 200g followed by 14,000g for 10minutes.
RNA purity
RNA samples were checked for purity using a NanoDrop™ UV Spectrophotometer (Thermo Scientific; Waltham, Massachusetts). A 260/280 ratio reading in the range of 1.8 to 2.2 was indicative of the purity of the RNA.12
Quantitative real time polymerase chain reaction
Quantitative real-time PCR was used to measure glutaminase (GLS) mRNA expression with the iQ5™ thermocycler (Biorad; Hercules, California) programmed as follows: Cycle 1, once at 50°C for 10minutes; Cycle 2, once at 95°C for 5 minutes; Cycle 3 (40 times), step 1 95°C for 10seconds; step 2, 58°C for 30seconds; Cycle 4, once at 95°C for 1 minute; Cycle 5, once at 55°C for 1 minute; Cycle 6, 81 cycles from 55-95°C for 10 seconds, Cycle 7, ∞ at 4 °C. Messenger RNA (mRNA) levels were measured from DRG at 12, 24, 48 and 96 hours and melt curves and threshold cycle (Ct) data generated. The GLS expression level of rat sacral DRG was normalized with that of rat β-actin mRNA. Primers used for RT-q PCR were as follows: primer set #1 (236bp) 5’-GCATTTCATGTTGGTCTTCC-3’ and 5’-ACCCTTTGATCACCTCCTTC-3’; primer set #2 (232bp) 5’-CTGAGGAAGATGAGCCAAAA-3’ and 5’-AGATACCTGGAATGCCACAA-3’ for rat glutaminase (GLS) (GenBank: NM_22586); (272bp) 5’-CACACTGTGCCCATCTATGA-3’ and 5’-CCGATAGTGATGACCTGACC-3’ for rat β-actin (BA) (GenBank: NM_031144) (Real-time Primers; Elkins Park, Pennsylvania).
Immunohistochemistry
Harlan Sprague-Dawley rats (n=12) were deeply anesthetized at 24 and 72 hours post-incision with tribromoethanol (2.5% w/v) (Sigma-Aldrich; Saint Louis, Missouri) and xylazine (100mg/ml) (Lloyd Laboratories; Walnut, California) and perfused through the ascending aorta with 75mL calcium-free Tyrode’s solution (pH 7.3), followed by 325mL paraformaldehyde/picric acid fixative.8 S1 DRG tissue was collected, post-fixed for 4 hr and incubated in 10% sucrose in phosphate buffered saline (PBS), pH 7.4, overnight at 4°C. DRG tissue sections were cut at 10μm with a cryostat (Leica Microsystems; Buffalo Grove, Illinois) and mounted onto gel coated ProbeOn™ (Fisher Scientific; Waltham, Massachusetts) slides and dried for 60-90min at 37°C. The tissue was rinsed inside slide mailers (5 slides per mailer) three times with PBS, pH 7.4 and placed onto a rocker for 10 minutes during each wash. The primary anti-serum for GLS was rabbit anti-GLS (1:10,000; Curthoys, Colorado State Univ.).13 The primary anti-serum for VGluT2 was rabbit anti-VGluT2 (1:2000; HY-19; Sigma-Aldrich; Saint Louis, Missouri). The anti-sera were placed into a blocking agent consisting of polyvinylpyrollidone (PVP), 0.5% bovine serum albumin (BSA) in PBS with Triton X-100 (PBS-T), pH 7.4 and tissues were incubated in antisera on a rocker in a cold room (4°C) for 72hours.8 The tissues were rinsed three times for 10 minutes each with PBS and placed on a rocker during each rinse. AlexaFluor® 488 conjugated goat anti-rabbit anti-serum (1: 2000) (Invitrogen; Grand Island, New York) was placed into PBS-T, pH 7.4 and tissues were incubated on a rocker for 60minutes at room temperature. The secondary anti-sera were decanted; the tissues were washed three times for 10minutes each with PBS (pH 7.4) and placed on a rocker during washes. ProLong Gold™ (Invitrogen; Grand Island, New York) mounting medium was added to each section of tissue and cover slips applied. Slides were stored at room temperature for 24hours, in a dark area, to allow for complete polymerization. Images were acquired using the 40X objective on an Olympus BX51 epifluorescence microscope (Olympus; Waltham, Massachusetts) equipped with a SPOT RT740 quantitative camera (Diagnostic Instruments; Sterling Heights, Michigan). To ensure proper quantitation, all images were captured using a 300-millisecond exposure with the gain set at 1. Captured images were 1600´1200 pixels with 1.9 pixels per micrometer. Four slides were used for each of the two antisera, GLS and VGluT2. There were a total of 360 images, 180 per antisera, 15images on each of 4 slides. All neuron profiles with a clearly visible nucleus that were not touching the edge of the image were traced on a Cintiq 12WX interactive pen display (Wacom; Vancouver, Washington) using the freehand selections tool in ImageJ (NIH; Bethesda, Maryland). Nuclei were excluded from the regions of interest (ROIs), making each ROI correspond to the cytoplasmic profile of a single DRG neuron. Since all images were acquired at a bit depth of 8, pixel intensities could range from 0 (darkest) to 255 (lightest) on a grayscale. The mean gray value and area in mm2 for each cytoplasmic profile was measured and copied to a spreadsheet. The values for area (µm2) were binned into small (<400µm2), medium (400-799.99µm2) and large (>800µm2) size categories to indicate three broadly defined neuronal subpopulations that correspond to C-, Aδ and Aα/β fibers, respectively.9 The three populations of DRG neurons also have been identified morphologically by immune-labeling patterns.10
Absorption controls
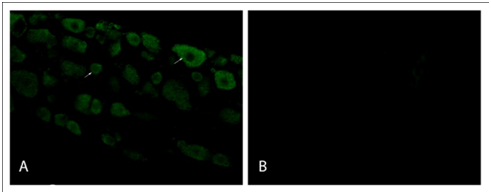
Primary antiserum absorption control and secondary antiserum control experiments were performed for the GLS and VGLUT2 primary antisera and secondary antisera, respectively. For primary antiserum absorption controls, each diluted antiserum was incubated for 24hours at 4°C with the respective antigen at 20mg/mL. Processing of DRG sections for immunofluorescence was carried out as mentioned above. Additional sections were incubated for 72hours at 4°C in either absorbed diluted antisera or non-absorbed diluted antisera. The secondary antisera control sections were incubated in PBS-T in the absence of primary antisera for 72hours at 4°C before continuing with routine processing (Figure 1).
Immunoblot
Harlan Sprague-Dawley rats (n=24) underwent CO2 asphyxiation and S1 DRG’s were obtained by rapid dissection at 12, 24, 72 and 96 hours post-incision. Right and left DRG tissues were combined and lysates were made from each sample using TPER lysis reagent (Pierce Chemical) containing EDTA, phosphatase, protease inhibitors (Pierce Chemical) and 300 mMNaCl to lyse the nuclear membrane. Samples were refrigerated at 2-8°C. Protein concentrations were quantified using the bicinchonic acid (BCA) protein assay kit (Pierce Chemical), as compared to a standard bovine serum albumin (BSA) concentration curve. The standard curve was created by adding 10µL of a standard curve dilution to a clear 96-well plate. The DRG protein was added to the plate in duplicate and nanopure H2O was added to the wells. Lysis buffer was added to each well of the plate and incubated at 60°C for 20minutes. The absorbance was read in a colorimetric plate reader (Biorad; Hercules, California). Equal amounts (30μg or 50μg) of cellular protein were loaded onto the SDS-PAGE gels, subjected to electrophoresis at 120V, transferred to a nitrocellulose membrane using iBlot apparatus (Invitrogen; Grand Island, New York) for seven minutes, blocked in Superblock (Pierce; Dallas, Texas) and incubated with primary antibody overnight at 4°C. The primary anti-sera for GLS were rabbit anti-GLS (1:10,000; Curthoys, Colorado State Univ.).13 The primary anti-serum for VGluT2 was rabbit anti-VGluT2 (1:2000; Sigma-Aldrich; Saint Louis, Missouri). Membranes were washed three times in Tris-buffered saline (TBS) containing 0.25% polysorbate 20, pH 7.5 and incubated with the secondary antibody, AlexaFluor® 488 conjugated goat-anti-rabbit IgG (1:2000; Pierce 32260; Dallas, Texas). Membranes were washed three times, enhanced chemiluminescence (ECL) was added for five minutes and membranes washed three times in PBS. Membranes were imaged using a digital charge-coupled device (CCD) camera digital darkroom (Image-Station 4000; Kodak, New Haven, Connecticut). Anti-vinculin antibody (mouse - Sigma Chemical; product V9131; 1:4000; Saint Louis, Missouri) or anti-ß-actin antibody (mous-Cell Signaling Technologies; 1:4000; Danvers, Massachusetts) was used to evaluate all blots for loading controls.
Statistical analysis
Data from the analyses are reported as mean value with standard error of the mean (sem). Comparison between groups means was performed with either the Student’s t-test (2 groups) or ANOVA (>2 groups) to determine differences between incisional and control groups (Prism version 6.0, GraphPad Software Inc., La Jolla, California). In all analyses, p-values less than 0.05 were considered significant.
Sacral innervation of the area of surgical incision
To study the innervation of the surgical incision, dorsal root ganglia (DRG) neurons were labeled by injection of the retrograde tracer, Fluoro-Gold™, into the area of surgical incision. Cells were binned for area into small (<400µm2), medium (400-799.99µm2) and large (>800µm2) size categories to indicate three broadly defined neuronal subpopulations that correspond to C-, Aδ and Aα/β fibers, respectively.8–10 The mean grayscale intensity (MGI)±sem for Fluoro-Gold™ in injection only rats, small size neurons was 139.5±1.63 relative units (ru), medium size neurons was 135.8±1.90ru and large size neurons was 138.9±2.51 ru. For rats with incision and Fluoro-Gold injection, the MGI for small size neurons was 99.17±2.83 ru, medium size neurons was 91.29±1.91 ru, large size neurons was 94.42±1.64 ru. There was no significant difference or preferential distribution of fluorescence among neuron size within either group (i.e., injection only, incision plus injection) indicating an equal uptake of the retrograde tracer.
Co-localization of VGluT2 and Fluoro-Gold™
Synaptic vesicles containing vesicular glutamate transporters type 2 (VGluT2) occur predominately in small diameter (<400µm2) nociceptive neurons.14 To determine that nociceptive neurons innervate the site of surgical incision, the co-localization of VGluT2 immunoreactivity (ir) and Fluro-Gold™ fluorescence in sacral DRG neurons was examined at 72hours post-tail incision. Numerous DRG neurons had co-localization for both VGluT2 and Fluoro-Gold™ (Figure 2). Image analysis revealed a significant increase (46%; p<0.05) in VGluT2 MGI 72-hours post-tail incision for small diameter neurons (138.2±2.06 ru) when compared to control (no incision; 94.57±6.60ru).



GLS messenger RNA (mRNA)
The fold change of mRNA expression in sacral level DRG GLS decreased by -3.83, -3.38, -4.43 and -3.14 of constitutive levels at 12, 24, 48 and 96hours respectively representing a significant decrease (p<0.05) compared to controls at all time points (Figure 3).
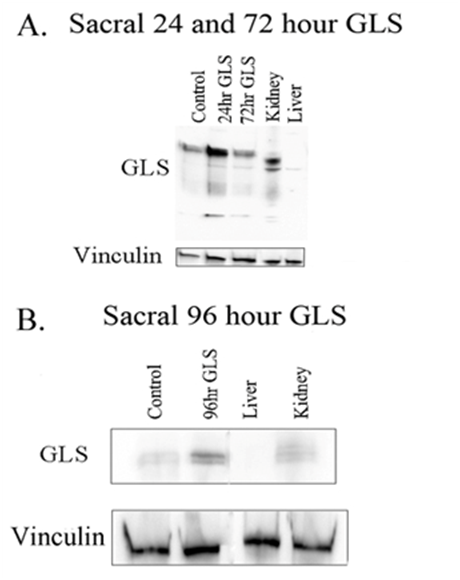
GLS immunoblot post-incision
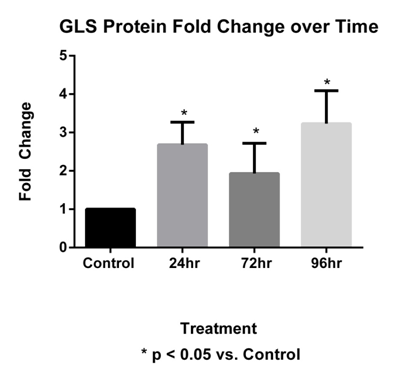
GLS expression was examined at 24, 72 and 96 hours post-tail incision (Figure 4). The sacral level DRG GLS protein 24, 72 and 96 hours increased by 2.68±0.59, 1.93±0.79 and 3.23±0.86 fold changes, respectively. All time points were significant, p <0.05 (Figure 5).
VGluT2 immunoblot post-incision
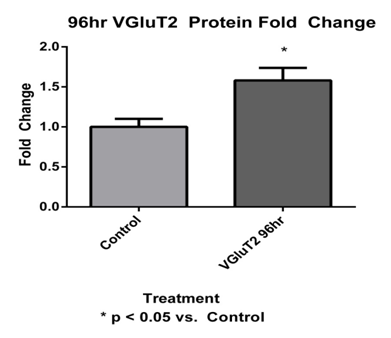
VGluT2 expression was examined at 96 hours post-tail incision (Figure 6). The sacral level DRG VGluT2 protein at 96 hours had significantly (p < 0.01) increased by a 1.87±0.29 fold change when compared to control VGluT2 (Figure 7).
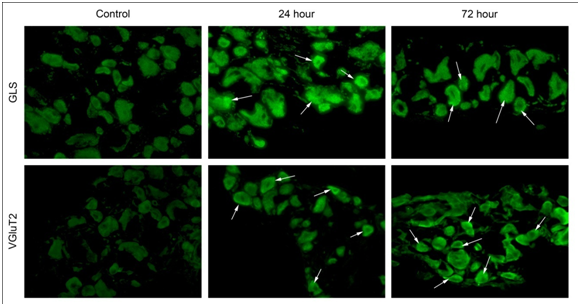
Immunohistochemistry (GLS and VGluT2) post-incision
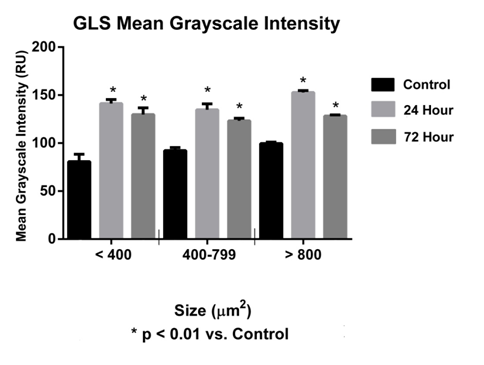
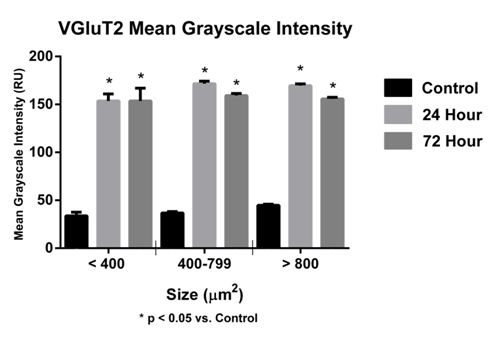
In this study, a significant increase (p<0.05) in GLS-ir in sacral DRG at 24 and 72hours was observed (Figure 8). The MGI for sacral DRG neurons (<400µm2) was 80.76±7.65 (control), at 24hours 141.20±4.26 (experimental) and at 72hours 129.80±6.93 ru (experimental). The MGI for sacral DRG neurons (400-799µm2) was 92.27±3.18ru (control), at 24hours 134.80±6.17 (experimental) and at 72hours 123.20±2.76ru (experimental). The MGI for sacral DRG neurons (>800µm2) was 99.62±1.40ru (control), at 24hours 152.80±1.94ru (experimental) and at 72 hours 128.30±1.22ru (experimental) (Figure 9). A significant increase (p<0.05) in sacral DRG VGluT2-ir at 24 and 72hours was observed (Figure 7) (Figure 9). The MGI for sacral level DRG neurons <400µm2 was 72.71±13.60ru (control), at 24 hours 147.30±5.68 ru (experimental), 72hours 153.60±13.24ru (experimental). The MGI for sacral level DRG neurons 400-799µm2 was 37.17±1.50ru (control), at 24hours 170.40±2.36 (experimental), 72 hours 157.10±1.89 ru (experimental). The MGI for sacral level DRG neurons >800µm2 was 45.03±0.97ru (control), at 24 hours 170.80±1.52 ru (experimental), 72 hours 154.10±1.48ru (experimental) (Figure 10).
More than 99 million surgeries take place annually in the United States. Over 80% of those patients who underwent surgery report postoperative pain, a type of acute pain and 86% of patients comment their pain ranges from moderate to extreme.15 Persistent post-surgical pain, a type of chronic pain that develops from acute post-operative pain, is a major clinical problem that affects up to 50% of patients, with 10% of patients rating the pain as moderate to severe.2 Furthermore, the effective control of post-operative pain has been described as a failing of our health care system.16 A surgical incision is an acute painful injury. Following incision, wound inflammation causes an increase in the production of nociceptive molecules in DRG neuronal cell bodies that augment both central and peripheral sensitization.17 These primary sensory neurons are glutamatergic, releasing glutamate from peripheral and central terminals via VGluT2 synaptic vesicles.14,18 Previous studies have demonstrated that glutamatergic metabolism is elevated in DRG neurons during adjuvant-induced arthritis (AIA), leading to hyperalgesia and allodynia.4,5 Our investigation was to evaluate if a similar alteration occurs after a surgical incision.
The tail incision model
To study post-operative pain, two main models have been used: the plantar incisional model developed by Brennan et al.,19 and the tail incision model developed by Weber et al.20 These models provide researchers the ability to test animal responses with numerous behavioral and electrophysiological tests. The tail incision model offers more neuronal DRG tissue for evaluation due to bilateral innervation compared to the unilateral innervation of the hind paw. The location of the tail incision offers a greater degree of protection of the incision site as well, compared to the hind paw model, as the animal does not ambulate on the incision and aggravation of the wound is minimized. Furthermore, the tail model offers several scientifically accepted behavioral tests (e.g., tail-flick, tail-flick using radiant heat, tail-flick using immersion, electrical stimulation of the tail, mechanical stimulation and suspension). In the current study, the incision was placed in the proximal tail, differing from Weber et al., a distal tail incision.20
Innervation of the incision area by sacral dorsal root ganglia afferents
To confirm the innervation of the incision area by sacral dorsal root ganglia neurons, the retrograde tracer, Fluoro-Gold™ (hydroxystilbamidine) was infiltrated into the area of incision and 72 hours later, sacral DRG neurons were analyzed for mean gray intensity (MGI). No difference in MGI was observed between the Fluoro-Gold™ control and incision groups, indicating a non-preferential labeling of neurons. Many DRG neurons with small diameter (<400µm2), lightly myelinated (A-δ) or unmyelinated axons (c-fibers), act as nociceptors and contain the glutamate transporter, VGluT2.14,21,22 In our retrograde study, VGluT2 was co-localized in sacral DRG neuronal cell bodies with Fluoro-Gold™ to validate labeling of small diameter neurons (<400µm2). Our findings confirm sacral innervation of the area of incision by small diameter neurons. Furthermore our examination showed that retrograde labeled, small diameter neurons in the experimental group had significantly increased VGluT2-ir post-surgical incision when compared to control groups 72hours post- tail incision, indicating an elevation in the amount of the glutamate transporter after incision. The results of the present study suggest that analysis of VGluT2-ir at the sacral level DRG in this surgical incision model can be useful as markers for analysis of nociception. These findings support previous work examining the innervation of the tail by sacral DRG projection neurons.23 Overall, these results help characterize and define a subpopulation of DRG neurons, which innervate the area of surgical incision and can be used to analyze neuronal changes post-surgical incision.
GLS messenger RNA (mRNA) changes in DRG neurons and in other systems
Surgical incision causes a consistent decrease in GLS mRNA in DRG when compared to surgical naïve animals. Sacral level DRG GLS mRNA had a significant decrease in fold change mRNA expression at 12, 24, 48 and 96 hours, respectively. During the same time period, there was a significant increase of GLS protein beginning at 12hours and remaining elevated for 96 hours. The relationship between mRNA and protein is not linear and the mechanisms of mRNA turnover and regulation still need to be defined. Rapid, sustained decreases in mRNA suggest the possibility of a pre-existing pool of GLS mRNA that is altered upon cell soma stimulation. This type of mechanism occurs in the regulation of kidney type GLS. Regulation of kidney type GLS during normal acid-base balance includes the weak binding of zeta (z)-crystallin to GLS mRNA for the rapid degradation of the GLS mRNA by a sequence specific endoribonuclease. During metabolic acidosis GLS mRNA is stabilized through the strong binding of zeta (z)-crystallin to an 8-base AU-rich pH-response element (pH-RE) of GLS mRNA. This type of mechanism allows kidney epithelial cells to adapt rapidly to changing conditions (e.g. metabolic acidosis).24 Further study is needed to relate and characterize these changes in GLS mRNA to the functional aspects of DRG neurons during surgical wounding.
Alterations in GLS protein during injury and inflammation
GLS is elevated in both the cytoplasm and mitochondria of primary afferent sensory neurons in the adjuvant-induced arthritis (AIA) model in the rat.4,5 During AIA, GLS shows a cytoplasmic elevation occurring between days 1 and 2, followed by an increase in the mitochondria between days 2-4. GLS enzyme activity, protein and ir remain elevated in the DRG from 2-8 days. When comparing the protein expression profiles in the aseptic incision to the inflammatory model, the expression profile is similar. The AIA model has a robust level of inflammation and swelling, but the aseptic incision model has components of inflammatory, nociceptive and neuropathic pain. In comparison the level of inflammation as described by swelling and redness may not be the only factor required to generate a sustained response in alterations of GLS mRNA and protein levels.
Alterations in VGluT2 protein during injury and inflammation
VGluT2 expression in primary afferent neurons plays an integral role in nociception, in both acute and chronic painful conditions.25,26 In the present study, VGluT2 expression was elevated in the sacral DRG similar to GLS. Overall, both immunoreactivity and immunoblot data showed increases in VGLuT2 within 24hours and lasting throughout 96hours. Immunohistochemical and western blot analysis of these studies confirm that both GLS and VGluT2, at the sacral level DRG in our surgical incision model, have increased expression. The elevation in GLS enzyme provides for an increase in glutamate production at both the spinal cord synapses and peripheral terminals. A sustained significant increase in sacral DRG GLS protein expression at all time points was observed; the expression level was not as great at the 72-hour time point. This correlates with the same pattern of GLS protein expression observed in the inflammatory model. Our lab offers the hypothesis that this decrease in GLS enzyme production is due to a change in the type of painful extracellular mediators stimulating the affected primary afferent neurons. In other words, during this time of decreased GLS production there is a change in the driving stimuli of the neuron, i.e., from painful cell derived mediators, (e.g. adenosine, prostaglandins, 5-hydroxytryptamine, bradykinin) to neurotrophic factors (e.g. NGF, BDNF).
In this study, the following hypothesis is addressed: If GLS is elevated in both nociceptive and neuropathic painful conditions, then GLS levels will be elevated in affected dorsal root ganglia (DRG), post-surgical incision. The overall experimental goal was to determine GLS mRNA and protein expression in sacral rat dorsal root ganglia post-incision, in vivo. In addition, VGluT2 was examined, as it is required for nociception in both acute and chronic pain.25,26
The observed increase in VGluT2 and GLS could provide an increase in overall glutamate function at spinal cord synapses and peripheral terminals, augmenting both central and peripheral sensitization, respectively. The change in production patterns of these proteins also implicates alteration in modality and function, (i.e., a ‘phenotypic switch’) and can provide surrogate markers for the analysis of nociception. Our results describe an interesting biological process that differs somewhat from the canonical mRNA/protein model, where a stimulus results in the increased production of mRNA followed by increased production of protein. In the current surgical incision model, tissue injury results in the use of an available pool of GLS mRNA to allow for the rapid translation of functional protein. Overall, results from these studies help further characterize the neuronal GLS and VGluT2 narrative during injury and offer potentially novel pharmacological target.
Authors have no conflicts of interest. This work was supported in part by the University of Oklahoma College of Pharmacy, Tulsa Community College Department of Biotechnology, Oklahoma State University Center for Health Sciences Intramural Grant and NIH Grant AR047410 (KM).
The author declares no conflict of interest.

©2015 Crosby, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.