eISSN: 2379-6367


Research Article Volume 4 Issue 6
1Division of Gynecologic Oncology, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Texas Health Science Center McGovern Medical School at Houston, USA
2UTHealth-Memorial Hermann Cancer Center-TMC, Houston, USA
3Division of Medical Oncology, Department of Internal Medicine, University of Texas Health Science Center McGovern Medical School at Houston, USA
4Department of Pharmacy, Memorial Hermann Hospital-TMC, USA
Correspondence: Judith A Smith, Associate Professor, Department of Obstetrics, Gynecology & Reproductive Sciences, Division of Gynecologic Oncology, UT Health McGovern Medical School, 6431 Fannin Street, Houston, USA , Tel 7135006408
Received: August 16, 2016 | Published: December 28, 2016
Citation: Burney M, Mosley S, Mosley AOMD, et al. Evaluation of potential cytochrome p450 and plasma protein binding drug interactions for the class of camptothecins. Pharm Pharmacol Int J. 2016;4(6):473-478. DOI: 10.15406/ppij.2016.04.00098
Purpose: To compare the potential metabolism and protein binding interactions with selected camptothecin agents.
Methods: Cytochrome P450 (CYP450) isoenzymes were used to screen and predict the enzymes involved in metabolism of each selected Camptothecin agent. Known substrates and inhibitors of each isoenzyme were used to predict drug interactions with the camptothecin agents. The effects of both albumin (Alb) and alpha-acidic glycoprotein (AAG) on plasma protein binding (PPB) for each camptothecin was assessed by equilibrium dialysis techniques in the presence of varying ratios of Alb and AAG.
Results: Karenitecin is metabolized by CYP3A4, 2D6, 2C8, and 2C9 and is an inhibitor of the 2D6 and 2C8 isoenzymes. Topotecan was primarily metabolized by 3A4 but also by 2D6 and 2C9. Irinotecan was similar to the parent compound, camptothecin, in its ability to inhibit 2D6 as well as being a substrate for 3A4. The mean percent protein bound was >85% for all agents evaluated with the exception of Topotecan whose protein binding was low yet highly variable with alterations in plasma protein concentration. The extent of camptothecin plasma protein binding was proportional to the plasma concentration of AAG.
Conclusion: The camptothecin agents have the potential for 3A4, 2C9, and 2C8 drug interactions that should be monitored prospectively to avoid toxicity. In addition, slight variations in plasma AAG and Alb concentration could result in large variations in free drug exposure and potentially contribute to increased toxicity. This should be monitored when employing combination chemotherapy with camptothecin agents.
Keywords: camptothecins, karenitecin, topotecan, irinotecan, cyp450, ppb
Camptothecin were first introduced in the early 1970’s, formulated in sodium hydroxide to enhance solubility, in clinical trials but was terminated due to a lack of clinical antitumor activity and a significant incidence of hemorrhagic diarrhea and uroepithelial toxicity.1 These unexpected toxicities are believed to have been due to instability of camptothecin, which exists as biologically inactive carboxylate salts in solution.2 In general, the development of camptothecin derivatives has been fraught with problems associated with the significant and unpredictable variability in the pharmacokinetics and pharmacodynamics of these compounds. This has been attributed to the limited water solubility of these drugs, variances in their metabolism and the relative instability of the active lactone form of these compounds mentioned.3
In the late 1990s, the cytotoxic nature of camptothecin was utilized for second line treatment of ovarian cancer and metastatic colon cancer and within the last decade camptothecin have been incorporated into many first-line regimens. The primary mechanism of action for camptothecin occurs through the binding of active lactone to Topoisomerase I, stabilizing it as a cleavable complex along the DNA replication fork. The formation of this complex results in an accumulation of single and double strand DNA breaks and ultimately apoptosis.4,5 Through this mechanism, camptothecin have demonstrated clinical activity in a variety of tumors and have already been incorporated into numerous combination regimens as well as continue to be actively evaluated for new indications.
Two of the major limitations of these agents have been the cumulative toxicity and inconsistent activity associated with camptothecin regimens. The potential drug interaction profile has to be defined to prevent unforeseen toxicity or potential decrease in activity. The objective of this study was to characterize the hepatic metabolism and plasma protein binding for the currently available FDA and EMEA approved camptothecin in clinical use, Topotecan and Irinotecan compared to the parent compound, camptothecin. Karenitecin, another camptothecin analog, was studied to validate its protein binding interactions which have been previously studied.6
A previous method, developed by Freeman BB et al.7 showed that the lower limit of quantitation for clinical phase I and phase II samples was appropriate for the analysis of drugs in plasma.7 Other studies have utilized this method to quantify drugs and gauge the pharmacokinetic profiles in plasma and cell lysates, including Topotecan.8,9 Four liver cytochrome genes, CYP2C8, CYP2C9, CYP3A, and CYP2D6, were selected based on high expression profile in liver and due to their prominent role in the biotransformation of the many drugs and foreign substances in clinical use.10 These isozymes were used to characterize the hepatic metabolism for the camptothecin. Previous work has shown that camptothecin had an attenuation effect on CYP3A4, with the exception of Irinotecan, which was shown to promote induction.11
Sepehri et al.12 utilized human serum albumin (HSA), also known as albumin (Alb) in this study, to overcome insolubility of camptothecin analog, Irinotecan and to improve active form stability.12 Another study by JA Smith et al utilized both Alb and alpha-acidic glycoprotein (AAG) to explore the protein-binding interactions of karenitecin.13 Thus Alb and AAG were used to describe the potential protein interactions of camptothecin analogs. Bom D and colleagues observed that modifications at the 7 or 9 position of the quinolone nucleus increase the binding affinity of the carboxylate form to albumin, thereby lowering the plasma lactone concentration.14 Also, AAG has also been showed to have an important role in the protein binding of camptothecin. Yaom S et al.15 measured lactone/carboxylate ratio of camptothecin analog, Karenitecin, after addition of Al and alpha-1 acid glycoprotein (AGP), also known as AAG. The results showed that AGP-bound Karenitecin enhanced lactone stability and had high lactone/carboxylate ratio. They explored the Karenitecin-protein binding using an AGP-immobilized column.15 Because of the increased throughput and minimal resource requirements of equilibrium dialysis compared to the column chromatographic protein techniques, our study utilized an equilibrium dialysis method.16
Chemicals and reagents
Camptothecin agents were purchased from vendors with the highest purity available. Camptothecin from Sigma Chemical Co. (St. Louis, MO, USA), Camptosar (Irinotecan hydrochloride injection) from Pfizer (New York, NY, USA), and Hycamtin (Topotecan hydrochloride injection) was purchased from GlaxoSmithKline (Brentford, Middlesex, UK).Karenitecin was generously provided by BioNumerik Pharmaceuticals, Inc. (San Antonio, TX, USA) Tris base was bought through Fisher (Pittsburgh, PA, USA). Quercetin, sulphaphenazole, ketoconazole, quinidine, dibenzylfluorescein (DBF), 3-[2-(N,N-diethyl-N-methylammonium)ethyl]-7-methoxy-4-methylcoumarin iodide (AMMC), acetonitrile, glucose-6- phosphate, glucose-6- phosphate dehydrogenase (G6PDH), tribasic sodium citrate, magnesium chloride hexahydrate, Nicotinamide adenine dinucleotide phosphate (NADP+), monobasic potassium phosphate (KH2PO4), sodium phosphate, sodium chloride, dextran, dibasic potassium phosphate (K2HPO4), and sodium hydroxide were all purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Sorensen’s solution was used as the dialysate for the plasma protein binding studies. It was prepare from 0.057 M (11.67g/L) potassium phosphate(KPO4), 0.067 M (9.51g/L) sodium phosphate, and 0.067 M (3.91g/L) sodium chloride dissolved in one liter deionized water(pH=7.4) at room temperature. Dextran 10 mg/mL was added to the Sorensen’s solution when carrying out equilibrium dialysis experiments. Cofactor solution for isoenzymes 3A4, 2C8, and 2C9 was composed as in previous study by Mach CM et al.17 The solution had final cofactor concentrations of NADP+ (20mg/mL), glucose-6-phosphate (20 mg/mL), magnesium chloride hexahydrate (13.3mg/mL). The stop solution was 2N NaOH (80mg/mL). CYP450 isoenzyme 2D6 cofactor solution was prepared identically, using a different concentration of NADP+ (1mg/mL) and a stop solution of 80:20 acetonitrile: 0.5 M tris base solution. Cofactors/serial dilution buffer for all isoenzymes were composed of 1.5mL 0.5M KPO4, 1.5mL cofactor solution (described above), 0.3mL G6PDH. Enzyme/substrate (E/S) solutions were unique for each isoenzyme and were prepared fresh for each experiment. Briefly the 3A4 E/S solution was composed of 2.94mL de-ionized waster, 7mL KPO4, 10µL DBF (2mM), and 50µL CYP3A4. 2C8 E/S solution was composed of 8.6mL of de-ionized water, 1 mL KPO4, 10µL DBF (2mM), and 400µL CYP2C8. 2C9 E/S solution was composed of 9.84mL de-ionized water, 10µL DBF (2mM), and 200µL CYP2C9. 2D6 E/S solution was composed of 6.9mL de-ionized water, 3mL KPO4, 3µL AMMC (10mM), and 150µL CYP2D6.
Cytochrome P450 enzyme microsomes
CYP450 3A4, 2C8, and 2C9 isoenzyme microsomes were purchased from BD Biosciences Gentest (Woburn, MA, USA). The total protein content is 5.8mg/mL in 100 mM potassium phosphate (pH 7.4) and corresponding CYP450 content is 1nmol/mL.
High throughput CYP450 inhibition assays
The assay protocol was modified from a validated high throughput method for measuring CYP450 Inhibition (version 4.2, 2000) method from BD Gentest (Woburn, MA, USA).18,19 Briefly, the test compounds (camptothecin, irinotecan, karenitecin, topotecan), positive controls (quercetin, sulfaphenazole, quinidine and ketoconazole), and substrates (DBF and AMMC) were made in 0.5 M potassium phosphate buffer, pH 7.4. Recommended manufacturer methods were followed to prepare the common and positive control solutions, cofactors stocks, and enzyme/substrate mixes. Each reaction well, with a final volume of 200µL, contained cofactor concentrations of 1.3mM NADP+, 3.3mM glucose-6-phosphate, and 0.4U/mL G6PDH, and 3.3mM magnesium ion. After adding the appropriate test compound and inhibitor positive control, the wells were serially diluted 1 to 3 for eight wells (all camptothecin agents range 5µM down to 0.00229µM).Then, appropriate enzyme/substrate solution was added to all wells. The reactions incubated at 37ºC for 30 to 60minutes, as required, and were stopped by addingstop solution. The plates were immediately analyzed with FL600 Fluorescence plate reader using specific wavelengths for each substrate/metabolite. For each experiment, control samples with a known amount of substrate and synthesized metabolite were prepared in the absence of the isoenzyme for qualitative comparisons. All experiments were performed in triplicate.
Plasma protein binding experiments
An equilibrium dialysis method was employed to evaluate the plasma protein binding properties of irinotecan, SN-38, topotecan, and karenitecin with varying concentrations of artificial plasma prepared from human albumin (Alb) and a-acidic gylcoprotein (AAG) dissolved in Sorenson’s phosphate buffer solution with 10% dextran. The ratio of Alb: AAG used ranged from 3g/dL to 5g/dL of ALB, and 50mg/dL to 300 mg/dL of AAG and was placed in sample chamber of the two-well equilibrium dialysis cell. Regenerated methylcellulose, 12-14 kDa MWCO, equilibrium dialyzer membrane discs were pre-soaked in Sorenson’s phosphate buffer 15-30minutes before use. Samples with varying Alb: AAG concentrations were spiked with 40ng/mL of irinotecan, SN-38, topotecan, and karenitecin, which were placed in assay chamber before dialysis. In addition, two control samples were prepared per experiment by spiking Sorenson’s PBS with the same concentration of irinotecan, SN-38, topotecan, and karenitecin. Five samples were prepared per concentration in each experiment. Experiments were repeated in triplicate. Samples incubated for at least 72hours at 37 ºC to reach equilibrium. Chamber volume was measured to correct for volume shifts. Free fraction of irinotecan, SN-38, topotecan, and karenitecin in lactone form as well as total drug (lactone+carboxylate) were assessed using HPLC with a previously published assay.19
Cytochrome P450 metabolism studies
In vitro CYP450 metabolism studies demonstrated that topotecan is a substrate of 3A4, 2C9, and 2D6 isoenzymes but did not demonstrate any inhibitory activity. Karenitecin was revealed to be a substrate of 3A4, 2D6, 2C8, 2C9, and an inhibitor of 2D6 and 2C8 isoenzymes. Irinotecan is a substrate of 3A4, and inhibitors of 2D6 isoenzymes. Camptothecin is a substrate of 3A4 and 2D6, and is an inhibitor of 2D6 isoenzyme (Table 1).
Substrate |
CYP450 3A4 |
CYP450 2D6 |
CYP450 2C8 |
CYP450 2C9 |
Topotecan |
S |
S |
- |
S |
Karenitecin |
S |
S/INH |
S/INH |
S |
Irinotecan |
S |
INH |
- |
- |
Camptothecin |
S |
S/INH |
- |
- |
Table 1 Summary of Camptothecins CYP450 Metabolism Profile
S: SubstrateI; NH: Inhibitor.
All of the agents assessed were substrates for the 3A4 isoenzyme. Karenitecin displayed affinity for all isoenzymes, acting as a substrate at 3A4, 2D6, 2C8, and 2C9 while inhibiting 2D6 and 2C8. Topotecan was metabolized by all enzymes with the exception of 2C8. Irinotecan was similar to the parent compound, camptothecin, in their ability to inhibit 2D6.
Plasma protein binding studies
Protein-binding studies confirmed all of the camptothecin agents analyzed were highly protein bound, >80%, in each ratio of protein concentrations used with the exception of topotecan whose binding varied highly across the spectrum of plasma protein concentrations from 17-44%.
The protein-bound fraction of irinotecan appeared dependent upon the plasma protein concentration. As Alb or AAG concentration increased, protein-bound irinotecan concentration increased with a mean percentage bound increasing from 81.1% at the lowest protein concentrations (Alb 2g/mL, AAG 50mg/mL) to 92.9% at highest concentration (Alb 5g/mL, AAG 300mg/mL) (Figure 1) (Figure 2). This decrease in free drug fraction was seen as the concentration of either plasma protein was increased independent of the other, as well as in combination.

Figure 1 Percent camptothecins protein bound with the varying Alb concentrations. The percent of protein bound of each of the compounds tested was evaluated by equilibrium dialysis. The ratio of Alb:AAG used ranged from 3 g/dL to 5 g/dL of ALB, and 50 mg/dL to 300 mg/dL of AAG. The concentration of camptothecins was determined by HPLC. Five samples were prepared per concentration in each experiment. Experiments were repeated in triplicate. This figure represents the mean percent plasma protein bound across the studied range of Alb.
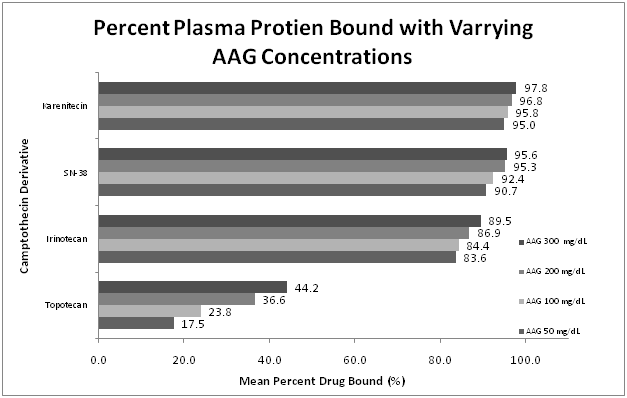
Figure 2 Percent camptothecins protein bound with the varying AAG concentrations.
The percent of protein bound of each of the compounds tested was evaluated by equilibrium dialysis. The ratio of Alb: AAG used ranged from 3 g/dL to 5 g/dL of ALB, and 50 mg/dL to 300 mg/dL of AAG. The concentration of camptothecins was determined by HPLC. Five samples were prepared per concentration in each experiment. Experiments were repeated in triplicate. This figure represents the mean percent plasma protein bound across the studied range of AAG.
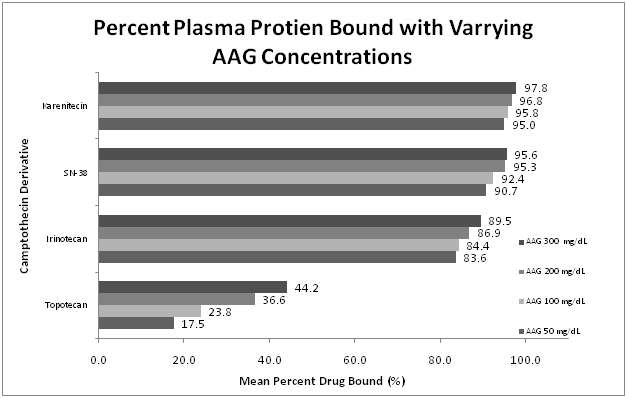
Figure 3 Change in percent bound with varying AAG and Alb concentrations.
The effect of differing concentration of plasma proteins was highly variable with the agents evaluated in this study. The change in bound percent of karenetecin and topotecan were highly dependent on AAG concentration. Irinotecan and SN-38 showed greater variability with alterations in Alb concentration.
Percent protein-bound karenitecin increased slightly as Alb and AAG concentration increased ranging from 95.9%-97.1%, with a mean of 96.3%. Of the camptothecins evaluated, karenitecin displayed the highest affinity for the plasma proteins. Because of this high affinity, the variability in percent bound was smallest for this agent, however, it equates to significant changes in the active free fraction which would translate into an increase in potential activity and/or toxicity.
Mean percentage of bound SN-38, a camptothecin metabolite, increased with rising plasma protein concentration as well. Figure 3 At the lowest concentrations of Alb and AAG (2g/mL and 50mg/mL respectively) the bound SN-38 was 87.0%. Contrast that with 96.1% at the highest concentration (Alb 5g/mL, AAG 300mg/mL).
Topotecan was the most variable agent evaluated and also showed the least affinity for either plasma protein. When studied across the range of rising Alb concentrations, the bound fraction increased from a mean percent bound of 23.5% to 37.7%. A greater difference was seen with the rising concentrations of AAG (mean percent bound ranging from 17.5% to 44.2%) suggesting that plasma protein binding of topotecan is more dependent on AAG concentrations than Alb.
Cancer patients are at a high risk for drug related adverse events due to the nature of their disease, the presence of co-morbid conditions, and the complexity of their medication regimens. In addition to the chemotherapy they receive, many patients rely on multiple drugs to manage the toxicities of that chemotherapy as well as medications for coagulopathy, depression, resulting infections, and chronic pain. The camptothecin agents have demonstrated in these studies two potential modalities by which the likelihood of adverse events could be increased. The agents in this class, including the commonly used irinotecan and topotecan, are dependent upon the CYP450 enzyme system for metabolism. In addition, camptothecins also display high percent plasma protein binding, hence, they are sensitive to even minor changes in the plasma protein concentration. A drug that ranges from 80 to 90% protein bound with altered protein concentrations will effectly double in free fraction dose in the presence of low levels of proteins. This is especially important, in this study, for karenatecin, SN-38, and irinotecan which all displayed a high degree of plasma protein binding.
Through the high throughput CYP450 metabolism studies, the metabolic profile of the camptothecin class of topoisomerase I inhibitors was further elucidated. The data show that as a class the camptothecins are highly dependent on several CYP450 isoenzymes for metabolism, mainly the CYP3A4. All of the agents in this class were classified as a substrate for CYP3A4. Co-administration of any one of these agents with medications known to inhibit the CYP3A4 isoenzyme could produce increase plasma levels of the camptothecin and potentially increased adverse drug events. The class of camptothecin agents with the exception of topotecan inhibited CYP2D6. This may also lead to clinically relevant interactions. Inhibition of this enzyme system may affect levels of antidepressants, opiods, and atypical anti-psychotics which may be used in conjunction with chemotherapy. Close monitoring for drug toxicities must be used in patients receiving these combinations of medications.
Karenitecin was the only agent that showed affinity for CYP2C8. This isoenzyme is responsible for the metabolism of warfarin as well as carbamazepine and phenytoin. Thus karenitecin’s inhibition of CYP2C8 would result in increased levels of these narrow therapeutic index medications and potential for clinically significant toxicities. Both topotecan and karenitecin are substrates of CYP2C9. This enzyme is also affected by aprepitant, resulting in enzyme induction. This again shows the necessity for caution when using aprepitant in combination with the camptothecins.
Irinotecan, karenitecin, and the parent compound camptothecin, were highly bound to both AAG and Alb. Changes in the concentration of these plasma proteins resulted in changes in the free fraction of drug within the plasma. While the percent bound to plasma protein only varied <5% for karenitecin and the active metabolite SN-38, and <15% for Irinotecan, this can cause alteration in the free fraction of 50-200% and can produce significant toxic effects in some patients.
Topotecan free drug concentrations were significantly altered by changes in both AAG and Alb concentrations. Although it displayed the lowest affinity for the plasma proteins studied, the variations in free fraction of Topotecan were the most significant and could affect plasma concentrations by as much as 25%. In patients with low serum protein levels, a dose adjustment may be necessary to decrease the likelihood of toxicity without decreasing the drug concentration at the tumor site.
It is worthy to note that the free drug concentration of some of the camptothecin was more influenced by changes in AAG concentration when compared to changes in Alb concentrations. This concept would suggest an increased clinical impact and need for more routine testing of AAG levels in patients who are to be given highly plasma protein bound drugs. As an acute phase reactant, AAG levels can fluctuate based on the patients clinical condition and may warrant dosage adjustment. AAG also displays a much shorter half-life than that of Alb and could increase or decrease within a cycle of chemotherapy.
The potential for both CYP450 and PPB drug interactions should be monitored closely when employing combination chemotherapy with camptothecin agents as well as co-administration with other commonly used drugs in cancer patients.
This study was partially supported by unrestricted research grant from BioNumerik Pharmaceuticals.
Author declares that there is no conflict of interest.

©2016 Burney, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.