eISSN: 2379-6367


Research Article Volume 8 Issue 1
1Chair and Department of Pharmaceutical Chemistry, Poznan University of Medical Sciences, Poland
2Chair and Department of Biophysics, School of Pharmacy and Laboratory Medicine, Medical University of Silesia in Katowice, Poland
3Chair and Department of Chemical Technology of Drugs, Poznan University of Medical Sciences, Poland
Correspondence: Anna Juszczak, Chair and Department of Pharmaceutical Chemistry, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland, Tel 0048601807130
Received: December 30, 2019 | Published: January 30, 2020
Citation: Juszczak A, Ramos P, Szczolko W, et al. Evaluation of antioxidant properties of angiotensin-converting enzyme inhibitors-interactions with free radicals model examined by EPR spectroscopy. Pharm Pharmacol Int J. 2020;8(1):25-32. DOI: 10.15406/ppij.2020.08.00276
Angiotensin-converting enzyme inhibitors (ACE-I) are the most popular drugs used in the modulation of renin-angiotensin-aldosterone system (RAS) activity. ACE-I show variable and complex biological activities, which determine the diversity of possible clinical use. Since it is known, that oxidative stress is the precursor of many pathologies and disorders, the antioxidant activity of drugs has emerged as a pharmacologically significant factor. There are a lot of clinical reports that intake of ACE-I improve conditions of patients with neurodegenerative disorders and may slow inflammatory processes. The aim of the study was a comparative analysis of antioxidant properties of the cilazapril, ramipril, imidapril, lisinopril, perindopril, and quinapril. EPR spectroscopy was used to examine chosen ACE-I interactions with the free radical model. Amplitude (A) of EPR lines of DPPH (reference), and DPPH interacting with the tested ACE-I were compared. Kinetics of interaction for tested ACE-I with DPPH, up to 30 minutes, was obtained. The most substantial interaction with DPPH was observed for cilazapril and the lowest for lisinopril. Studies have shown usefulness EPR spectroscopy for investigation interactions of ACE-I with the free radical model.
Keywords: angiotensin-converting enzyme inhibitors (ACE-I), antioxidant, free radicals, EPR spectrometry, UV-Vis spectrophotometry.
The renin-angiotensin system (RAS) is responsible for cardiovascular, renal and adrenal homeostasis and the physiological maintenance of blood pressure. The angiotensin-I converting enzyme (ACE) is a crucial enzymatic component of this system, and its main role is the conversion of angiotensin I (Ang I) to angiotensin II (Ang II). When RAS works deficiently and cannot regulate the balance between Ang I and Ang II, this reaction can be targeted by a well-known group of drugs - ACE inhibitors (ACE-I). ACE-I were developed as antihypertensive agents, and the effects of ACE-I on the RAS are well documented.1‒6 However, there are more and more reports about numerous beneficial consequences resulting from their pleiotropic activity which are clinically relevant. ACE-I have been extensively described as useful in the treatment of hypertension, but also as drugs delaying progression in diabetic nephropathy and reducing mortality in left ventricular dysfunction and congestive heart failure.7‒16 What is less obvious, ACE-I are also widely used in pediatric nephrology and cardiology.17‒25
Antioxidant activity has emerged as a pharmacologically important factor since oxidative stress is known as the precursor of many pathologies and disorders.39‒45 Studies proved that patients with renal and cardiovascular diseases have a lower level of tissue antioxidants and using ACE-I decreased vascular inflammatory markers, reducing coronary atherosclerosis.46,47 The role of anti-inflammatory and antioxidant mechanisms of ACE-I-induced action is also widely discussed in the modulation of rheumatoid arthritis,48 hepatitis,49 nephropathy,50 and retinopathy risk reduction.51 Moreover, there are more and more reports showing evidence that ACE-I can be beneficial in the treatment of neurodegenerative diseases since centrally active ACE-I can exert some anti-inflammatory effects, and they may counteract this cerebral pro-inflammatory state. Observational studies lead to a conclusion that brain-penetrating ACE-I slow the rate of cognitive decline in Alzheimer’s disease.52 Parkinson’s disease progression also can be triggered by oxidative stress. ACE-I active in the central nervous system also may serve as Parkinson’s disease treatment.29‒31,53,54 That neuroprotective activity of ACE-I is mainly based on their antioxidant properties, which is related to their ability to suppress the activity of NADPH oxidase, an enzyme responsible for ROS generation and the production of proinflammatory mediators.26‒38
As all the reports about antioxidant properties of ACE-I come from clinical investigations or mice models, therefore the study aimed to determine the percentage of inhibition of free radicals donor – DPPH (2,2-diphenyl-1-picrylhydrazyl). Electron paramagnetic resonance (EPR) spectroscopy and was used to test the antioxidant properties of ACE-I by its interaction with the free radical model.
Samples characterization
Tested ACE-I were: cilazapril, ramipril, imidapril, lisinopril, perindopril, and quinapril. Chemical structures of the tested drugs are shown in Table 1.55 Clinically, the potency of the individual family members is not equal, and this phenomenon results from their heterogeneity dependent on: structure, lipophilicity profile, pharmacokinetics, affinity to various forms of ACE (plasma, tissue) and the administration form (active drug or pro-drug)–Table 1.
ACE-I/ its active form |
Structure |
tmax [h] |
LogP* |
IC50 [nM] |
cmax [ng/L] |
fb [%] |
F [%] |
BBB |
Imidapril/Imidaprilat |
7-Feb |
-0.23 |
1.7 |
34.7 / 20.4 |
85 / 53 |
70 / 42 |
No |
|
Lisinopril |
7 |
-3.1 |
1.2 |
38 |
10 |
25 |
Yes |
|
Perindopril/Perindoprilat |
2/2/2004 |
0.63 |
1.5 |
116 |
20-Oct |
66 |
Yes |
|
Quinapril/ Quinalaprilat |
0.63 |
1.96 |
2.8 |
579 |
97 |
37 |
No |
|
Ramipril/Ramiprilat |
0.7 / 2.1 |
1.47 |
2 |
52.2 / 33.6 |
73 / 56 |
28 / 44 |
No |
|
Cilazapril/Cilazaprilat |
0.83 / 1.67 |
-1.01 |
1.9 |
81.8 / 36.2 |
30 |
77.5 / 29 |
Yes |
Table 1 Physical and pharmacokinetic properties of selected ACE-I: tmax–time to reach peak serum concentration, cmax–maximum serum concentration, fb–protein binding, F–bioavailability, BBB-penetrating the blood-brain barrier (2,5,7,56-60)
*administration form (pro-drug/active form)
EPR examination of interactions of ACE-I with free radicals
Interactions of ACE-I with free radicals were examined by the use of paramagnetic reference DPPH (2,2-diphenyl-1-picrylhydrazyl) model containing free radicals with unpaired electrons located on nitrogen (N) atoms. Chemical structure of DPPH and localization of unpaired electrons is shown in Figure 1.60‒63 DPPH was purchased from the Sigma-Aldrich company.

Figure 1Chemical structure of DPPH (2,2-diphenyl-1-picrylhydrazyl) molecule. (•) – unpaired electron (60-63).
EPR tested samples were prepared by mixing 1.5ml of the ethanolic solution of DPPH (c=0,5mM) and 50mg of each ACE-I. Samples were mixed and placed in thin-walled measuring glass tubes with an external diameter of 1mm. EPR spectra were not observed in empty glass tubes. To examine interactions of ACE-I with free radicals EPR spectra of free radicals of DPPH were measured without microwave saturation at the low microwave power of 2.2mW. The total microwave power produced by klystron was 70mW. The dependence of microwave power on attenuation is expressed by following formula:63,64
Attenuation [dB] = 10 lg (M/Mo)
Where:
M – microwave power,
Mo – total microwave power (70mW).
Microwave frequency was detected by MCM 101 recorder (EPRAD Company, Poznan, Poland). The EPR spectra were measured as the first-derivative line. The EPR spectra were measured at room temperature by the use of the electron paramagnetic resonance (EPR) an X-band (9.3 GHz) (Radiopan Company, Poznan, Poland). EPR measurements and analysis were performed using spectroscopic programs LabView 8.5 (National Instruments Company, Texas, USA) and SWAMP (Jagmar Company, Krakow, Poland). Amplitudes (A) of the EPR spectra of DPPH in ethyl alcohol solution-Figure 2 and amplitudes (A) of EPR lines of DPPH with ACE-I were determined.
Amplitudes (A) of EPR lines of DPPH after addition of the tested ACE-I samples decreased. This decrease reflected interactions of ACE-I with free radicals of DPPH. Kinetics of these interactions were examined up to 30 minutes. The changes of amplitudes (A) of EPR lines of DPPH interacting with ACE-I were measured up to 30 minutes every 3 minutes. The g-factor [+0.0002] characterized the type of free radicals and localization of unparsed electrons.65,66 The g-factor [+0.0002] for DPPH EPR lines was determined from the resonance condition according to following formula:65‒69
g = hν/μBBr
where:
h–Planck constant,
ν–microwave frequency,
μB–Bohr magneton,
Br–induction of resonance magnetic field.
Result of EPR spectroscopy examination
Interactions of ACE-I with free radicals were confirmed. EPR spectra of the free radical model of DPPH were quenched by cilazapril, imidapril, perindopril, ramipril, quinapril, and lisinopril. The quenching of EPR spectra of DPPH by ACE-I are shown in Figure 3. EPR spectra of DPPH (Figure 4a-f) interacting with the tested ACE-I during 3, 12, 21, and 30 minutes are compared.
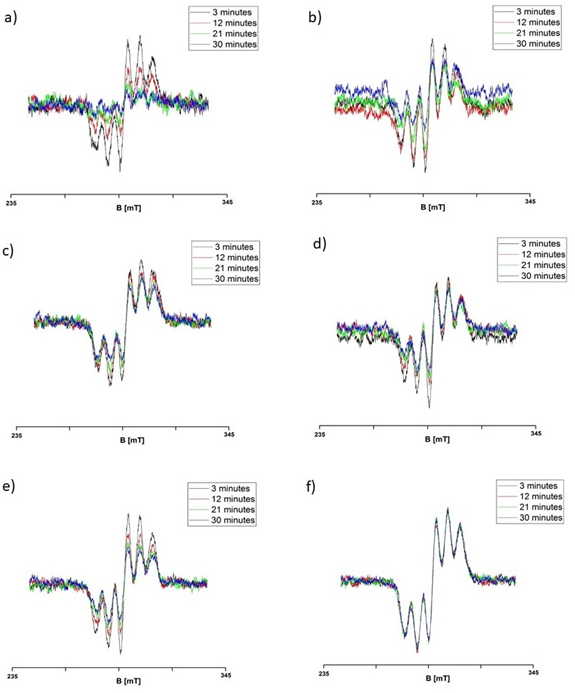
Figure 3 EPR spectra of DPPH interacting with (a) cilazapril, (b) imidapril, (c) perindopril, (d) ramipril, (e) quinapril, and (f) lisinopril during 3, 12, 21, and 30 minutes. B – magnetic induction.
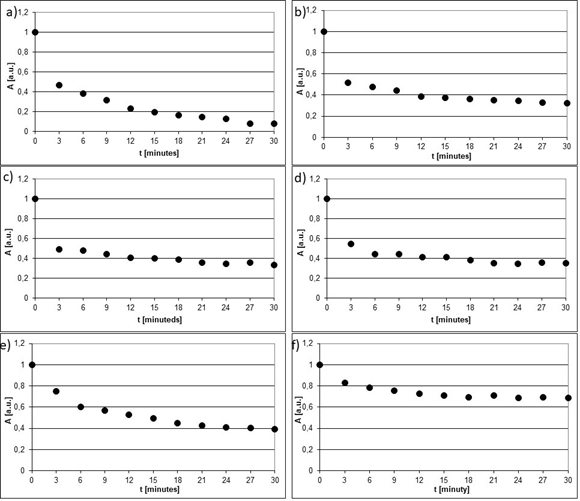
Figure 4 Changes of amplitudes (A) [+0.01 a.u.] of EPR spectra of DPPH interacting with (a) cilazapril, (b) imidapril, (c) perindopril, (d) ramipril, (e) quinapril, and (f) lisinopril with increasing interaction time (t).
The kinetics of quenching of EPR lines of DPPH by cilazapril, imidapril, perindopril, ramipril, quinapril, and lisinopril are presented in Figure 4a-f. Amplitudes (A) of EPR lines of DPPH decreased with increasing time of interaction with ACE-I and they achieved constant values after 27 minutes for cilazapril (Figure 4a), after 24 minutes for imidapril (Figure 4b), after 21 minutes for perindopril (Figure 4c), after 21 minutes for ramipril (Figure 4d), after 24 minutes for quinapril (Figure 4e), and after 15 minutes for lisinopril (Figure 4f) respectively. The all tested drugs quenched EPR lines of DPPH (Figure 3a-f). These relations are visible from the comparison of the lowest values of amplitudes (A) in Figure 4a-f, which were marked as the horizontal lines.
The tested drugs differed in scavenging activity of the free radical model (DPPH). Their ability to scavenging the free radicals was compared to L-ascorbic acid (from the Sigma-Aldrich Company), which is known as one of the most active antioxidants. Amplitude (A) of EPR spectra of DPPH proved that the interactions of the tested ACE-I with DPPH decreased in the fooling order: cilazapril > imidapril > perindopril > ramipril > quinapril > lisinopril and are shown in Table 2 and Figure 5.
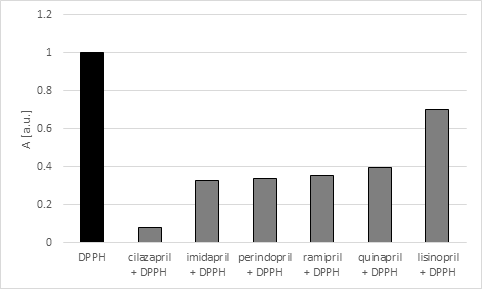
Figure 5 Comparision of the amplitudes (A) [+0.01 a.u.] of EPR line of DPPH interacting with the tested ACE-I after 30 minutes of the interaction.
test sample |
% inhibition [+1%] |
L-acorbic acid* |
100 |
cilazapril |
92 |
imidapril |
67.5 |
perindopril |
66.5 |
ramipril |
64.5 |
quinalapril |
60.7 |
lisinopril |
30 |
Table 2 Calculated % inhibition for ACE-I after 30 minutes incubation. For comparison, the L-ascorbic acid test was tested in the same conditions (measured in the same proportions as for ACE-I)
The% inhibition was calculated using the formula:
%inh. = ADPPH – Asamp. /ADPPH x 100
Where:
ADPPH-amplitude (A) of the standard alcoholic DPPH solution (c=0,5mM)
Asamp-amplitude (A) of the standard alcohol DPPH solution interacting with the tested ACE-I at the 30th minute of the measurement.
Hypertension is one of the most common diseases all over the world, and antihypertensive drugs are the most frequently prescribed group of medicines, it should be taken into account that using ACE-I has many advantages besides hypertension treatment, especially for patients in advanced age and with comorbidities. Their beneficial actions such as cardio-protection, vaso-protection, reno-protection or cerebro-protection represent a high added value to their primary hypotensive effect thanks to which they finally became the key to the neurohormonal treatment of cardiac insufficiency. Furthermore, the continually increasing understanding of the biophysical and pharmacological features can still provide new opportunities for extending their already broad clinical application.
EPR examination of ACE-I indicated that:
This work was financially supported by Medical University of Silesia in Katowice, grant number: KNW-1-059/K/7/0.
Authors declare that there is no conflict of interest.

©2020 Juszczak, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.